Mid-temperature CO2 Adsorption over Different Alkaline Sorbents Dispersed over Mesoporous Al2O3
- PMID: 38496972
- PMCID: PMC10938334
- DOI: 10.1021/acsomega.3c07204
Mid-temperature CO2 Adsorption over Different Alkaline Sorbents Dispersed over Mesoporous Al2O3
Abstract
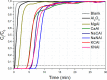
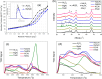
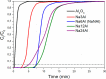
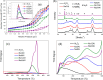
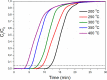
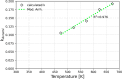
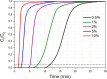
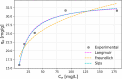
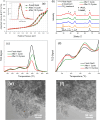
CO2 adsorbents comprising various alkaline sorption active phases supported on mesoporous Al2O3 were prepared. The materials were tested regarding their CO2 adsorption behavior in the mid-temperature range, i.e., around 300 °C, as well as characterized via XRD, N2 physisorption, CO2-TPD and TEM. It was found that the Na2O sorption active phase supported on Al2O3 (originated following NaNO3 impregnation) led to the highest CO2 adsorption capacity due to the presence of CO2-philic interfacial Al-O--Na+ sites, and the optimum active phase load was shown to be 12 wt % (0.22 Na/Al molar ratio). Additional adsorbents were prepared by dispersing Na2O over different metal oxide supports (ZrO2, TiO2, CeO2 and SiO2), showing an inferior performance than that of Na2O/Al2O3. The kinetics and thermodynamics of CO2 adsorption were also investigated at various temperatures, showing that CO2 adsorption over the best-performing Na2O/Al2O3 material is exothermic and follows the Avrami model, while tests under varying CO2 partial pressures revealed that the Langmuir isotherm best fits the adsorption data. Lastly, Na2O/Al2O3 was tested under multiple CO2 adsorption-desorption cycles at 300 and 500 °C, respectively. The material was found to maintain its CO2 adsorption capacity with no detrimental effects on its nanostructure, porosity and surface basic sites, thereby rendering it suitable as a reversible CO2 chemisorbent or as a support for the preparation of dual-function materials.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Olabi A. G.; Abdelkareem M. A. Renewable Energy and Climate Change. Renewable Sustainable Energy Rev. 2022, 158, 112111. 10.1016/j.rser.2022.112111. - DOI
-
- Kumar S.; Srivastava R.; Koh J. Utilization of Zeolites as CO2 capturing Agents: Advances and Future Perspectives. J. CO2 Util. 2020, 41, 101251. 10.1016/j.jcou.2020.101251. - DOI
LinkOut - more resources
Full Text Sources
