Where are the Excess Electrons in Subvalent Compounds? The Case of Ag7Pt2O7
- PMID: 38497133
- PMCID: PMC10988551
- DOI: 10.1021/acs.inorgchem.3c04409
Where are the Excess Electrons in Subvalent Compounds? The Case of Ag7Pt2O7
Abstract
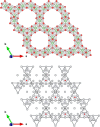
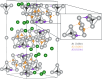
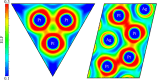
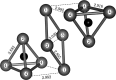
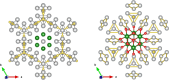
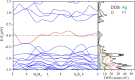
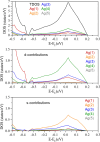
Subvalent compounds raise the question of where those valence electrons not belonging to chemical bonds are. In the limiting case of Ag7Pt2O7, there is just one-electron excess in the chemical formula requiring the presence of Ag atoms with oxidation states below +1, assuming conventional Pt4+ and O2- ions. Such a situation challenges the understanding of the semiconducting and diamagnetic behavior observed in this oxide. Previous explanations that localize pairwise the electron excess in tetrahedral Ag4 interstices do not suffice in this case, since there are six silver tetrahedral voids and only an excess of nine electrons in the unit cell. Here, we provide an alternative explanation for the subvalent nature of this compound by combining interatomic distances, electron density-based descriptors, and orbital energetic analysis criteria. As a result, Ag atoms that do not participate in their valence electron are revealed. We identify excess electrons located in isolated subvalent silver clusters with electron-deficient multicenter bonds resembling pieces of metallic bonding in fcc-Ag and Ag7Pt2 alloy. Our analysis of the electronic band structure also supports the multicenter bonding picture. This combined approach from the real and reciprocal spaces reconciles existing discrepancies and is key to understanding the new chemistry of silver subvalent compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Dedieu A.; Hoffmann R. Platinum (0)-platinum (0) dimers. Bonding relationships in a d10-d10 system. J. Am. Chem. Soc. 1978, 100, 2074–2079. 10.1021/ja00475a017. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

