The deubiquitinase function of ataxin-3 and its role in the pathogenesis of Machado-Joseph disease and other diseases
- PMID: 38497605
- PMCID: PMC11088879
- DOI: 10.1042/BCJ20240017
The deubiquitinase function of ataxin-3 and its role in the pathogenesis of Machado-Joseph disease and other diseases
Abstract
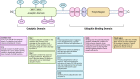
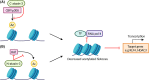
Machado-Joseph disease (MJD) is a devastating and incurable neurodegenerative disease characterised by progressive ataxia, difficulty speaking and swallowing. Consequently, affected individuals ultimately become wheelchair dependent, require constant care, and face a shortened life expectancy. The monogenic cause of MJD is expansion of a trinucleotide (CAG) repeat region within the ATXN3 gene, which results in polyglutamine (polyQ) expansion within the resultant ataxin-3 protein. While it is well established that the ataxin-3 protein functions as a deubiquitinating (DUB) enzyme and is therefore critically involved in proteostasis, several unanswered questions remain regarding the impact of polyQ expansion in ataxin-3 on its DUB function. Here we review the current literature surrounding ataxin-3's DUB function, its DUB targets, and what is known regarding the impact of polyQ expansion on ataxin-3's DUB function. We also consider the potential neuroprotective effects of ataxin-3's DUB function, and the intersection of ataxin-3's role as a DUB enzyme and regulator of gene transcription. Ataxin-3 is the principal pathogenic protein in MJD and also appears to be involved in cancer. As aberrant deubiquitination has been linked to both neurodegeneration and cancer, a comprehensive understanding of ataxin-3's DUB function is important for elucidating potential therapeutic targets in these complex conditions. In this review, we aim to consolidate knowledge of ataxin-3 as a DUB and unveil areas for future research to aid therapeutic targeting of ataxin-3's DUB function for the treatment of MJD and other diseases.
Keywords: deubiquitinase; neurodegeneration; polyglutamine repeat; spinocerebellar ataxia type-3; ubiquitin proteasome system.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Toward therapeutic targets for SCA3: Insight into the role of Machado-Joseph disease protein ataxin-3 in misfolded proteins clearance.Prog Neurobiol. 2015 Sep;132:34-58. doi: 10.1016/j.pneurobio.2015.06.004. Epub 2015 Jun 27. Prog Neurobiol. 2015. PMID: 26123252 Review.
-
Physiological and pathophysiological characteristics of ataxin-3 isoforms.J Biol Chem. 2019 Jan 11;294(2):644-661. doi: 10.1074/jbc.RA118.005801. Epub 2018 Nov 19. J Biol Chem. 2019. PMID: 30455355 Free PMC article.
-
ATXN3: a multifunctional protein involved in the polyglutamine disease spinocerebellar ataxia type 3.Expert Rev Mol Med. 2024 Sep 25;26:e19. doi: 10.1017/erm.2024.10. Expert Rev Mol Med. 2024. PMID: 39320846 Free PMC article. Review.
-
Machado-Joseph Disease: A Stress Combating Deubiquitylating Enzyme Changing Sides.Adv Exp Med Biol. 2020;1233:237-260. doi: 10.1007/978-3-030-38266-7_10. Adv Exp Med Biol. 2020. PMID: 32274760 Review.
-
Interaction of the polyglutamine protein ataxin-3 with Rad23 regulates toxicity in Drosophila models of Spinocerebellar Ataxia Type 3.Hum Mol Genet. 2017 Apr 15;26(8):1419-1431. doi: 10.1093/hmg/ddx039. Hum Mol Genet. 2017. PMID: 28158474 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

