Isolation of a recombinant simian adenovirus encoding the human adenovirus G52 hexon suggests a simian origin for human adenovirus G52
- PMID: 38497664
- PMCID: PMC11019922
- DOI: 10.1128/jvi.00043-24
Isolation of a recombinant simian adenovirus encoding the human adenovirus G52 hexon suggests a simian origin for human adenovirus G52
Abstract
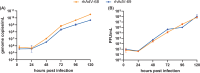
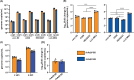
Human adenoviruses (HAdVs) are causative agents of morbidity and mortality throughout the world. These double-stranded DNA viruses are phylogenetically classified into seven different species (A-G). HAdV-G52, originally isolated in 2008 from a patient presenting with gastroenteritis, is the sole human-derived member of species G. Phylogenetic analysis previously suggested that HAdV-G52 may have a simian origin, indicating a potential zoonotic spillover into humans. However, evidence of HAdV-G52 in either human or simian populations has not been reported since. Here, we describe the isolation and in vitro characterization of rhesus (rh)AdV-69, a novel simian AdV with clear evidence of recombination with HAdV-G52, from the stool of a rhesus macaque. Specifically, the rhAdV-69 hexon capsid protein is 100% identical to that of HAdV-G52, whereas the remainder of the genome is most similar to rhAdV-55, sharing 95.36% nucleic acid identity. A second recombination event with an unknown adenovirus (AdV) is evident at the short fiber gene. From the same sample, we also isolated a second, highly related recombinant AdV (rhAdV-68) that harbors a distinct hexon gene but nearly identical backbone compared to rhAdV-69. In vitro, rhAdV-68 and rhAdV-69 demonstrate comparable growth kinetics and tropisms in human cell lines, nonhuman cell lines, and human enteroids. Furthermore, we show that coinfection of highly related AdVs is not unique to this sample since we also isolated coinfecting rhAdVs from two additional rhesus macaque stool samples. Our data collectively contribute to elucidating the origins of HAdV-G52 and provide insights into the frequency of coinfections and subsequent recombination in AdV evolution.IMPORTANCEUnderstanding the host origins of adenoviruses (AdVs) is critical for public health as transmission of viruses from animals to humans can lead to emergent viruses. Recombination between animal and human AdVs can also produce emergent viruses. HAdV-G52 is the only human-derived member of the HAdV G species. It has been suggested that HAdV-G52 has a simian origin. Here, we isolated from a rhesus macaque, a novel rhAdV, rhAdV-69, that encodes a hexon protein that is 100% identical to that of HAdV-G52. This observation suggests that HAdV-G52 may indeed have a simian origin. We also isolated a highly related rhAdV, differing only in the hexon gene, from the same rhesus macaque stool sample as rhAdV-69, illustrating the potential for co-infection of closely related AdVs and recombination at the hexon gene. Furthermore, our study highlights the critical role of whole-genome sequencing in understanding AdV evolution and monitoring the emergence of pathogenic AdVs.
Keywords: AdV; HAdV; adenovirus; human adenovirus; rhAdV; rhesus adenovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The genome sequence of a novel simian adenovirus in a chimpanzee reveals a close relationship to human adenoviruses.Arch Virol. 2014 Jul;159(7):1765-70. doi: 10.1007/s00705-013-1967-1. Epub 2014 Jan 8. Arch Virol. 2014. PMID: 24398862
-
Novel adenoviruses in wild primates: a high level of genetic diversity and evidence of zoonotic transmissions.J Virol. 2011 Oct;85(20):10774-84. doi: 10.1128/JVI.00810-11. Epub 2011 Aug 10. J Virol. 2011. PMID: 21835802 Free PMC article.
-
The possible origin of human adenovirus type 3: Evidence of natural genetic recombination between human and simian adenovirus.Infect Genet Evol. 2018 Nov;65:380-384. doi: 10.1016/j.meegid.2018.08.020. Epub 2018 Aug 23. Infect Genet Evol. 2018. PMID: 30144567
-
Human adenovirus type 8: the major agent of epidemic keratoconjunctivitis (EKC).J Clin Virol. 2014 Dec;61(4):477-86. doi: 10.1016/j.jcv.2014.10.015. Epub 2014 Nov 4. J Clin Virol. 2014. PMID: 25464969 Review.
-
Pathogenicity and virulence of human adenovirus F41: Possible links to severe hepatitis in children.Virulence. 2023 Dec;14(1):2242544. doi: 10.1080/21505594.2023.2242544. Virulence. 2023. PMID: 37543996 Free PMC article. Review.
Cited by
-
Adenovirus infections: new insights for the clinical laboratory.J Clin Microbiol. 2024 Sep 11;62(9):e0083622. doi: 10.1128/jcm.00836-22. Epub 2024 Aug 27. J Clin Microbiol. 2024. PMID: 39189703 Free PMC article. Review.
References
-
- Benkő M, Aoki K, Arnberg N, Davison AJ, Echavarría M, Hess M, Jones MS, Kaján GL, Kajon AE, Mittal SK, Podgorski II, San Martín C, Wadell G, Watanabe H, Harrach B, ICTV Report Consortium . 2022. ICTV virus taxonomy profile: Adenoviridae 2022. J Gen Virol 103:001721. doi:10.1099/jgv.0.001721 - DOI - PMC - PubMed
-
- HAdV working group. Available from: http://hadvwg.gmu.edu. Retrieved 7 May 2023.
-
- Abbink P, Kirilova M, Boyd M, Mercado N, Li Z, Nityanandam R, Nanayakkara O, Peterson R, Larocca RA, Aid M, Tartaglia L, Mutetwa T, Blass E, Jetton D, Maxfield LF, Borducchi EN, Badamchi-Zadeh A, Handley S, Zhao G, Virgin HW, Havenga MJ, Barouch DH. 2018. Rapid cloning of novel rhesus adenoviral vaccine vectors. J Virol 92:e01924-17. doi:10.1128/JVI.01924-17 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

