How much should we sequence? An analysis of the Swiss SARS-CoV-2 surveillance effort
- PMID: 38497714
- PMCID: PMC11064629
- DOI: 10.1128/spectrum.03628-23
How much should we sequence? An analysis of the Swiss SARS-CoV-2 surveillance effort
Abstract
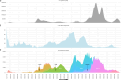
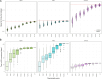
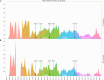
During the SARS-CoV-2 pandemic, many countries directed substantial resources toward genomic surveillance to detect and track viral variants. There is a debate over how much sequencing effort is necessary in national surveillance programs for SARS-CoV-2 and future pandemic threats. We aimed to investigate the effect of reduced sequencing on surveillance outcomes in a large genomic data set from Switzerland, comprising more than 143k sequences. We employed a uniform downsampling strategy using 100 iterations each to investigate the effects of fewer available sequences on the surveillance outcomes: (i) first detection of variants of concern (VOCs), (ii) speed of introduction of VOCs, (iii) diversity of lineages, (iv) first cluster detection of VOCs, (v) density of active clusters, and (vi) geographic spread of clusters. The impact of downsampling on VOC detection is disparate for the three VOC lineages, but many outcomes including introduction and cluster detection could be recapitulated even with only 35% of the original sequencing effort. The effect on the observed speed of introduction and first detection of clusters was more sensitive to reduced sequencing effort for some VOCs, in particular Omicron and Delta, respectively. A genomic surveillance program needs a balance between societal benefits and costs. While the overall national dynamics of the pandemic could be recapitulated by a reduced sequencing effort, the effect is strongly lineage-dependent-something that is unknown at the time of sequencing-and comes at the cost of accuracy, in particular for tracking the emergence of potential VOCs.IMPORTANCESwitzerland had one of the most comprehensive genomic surveillance systems during the COVID-19 pandemic. Such programs need to strike a balance between societal benefits and program costs. Our study aims to answer the question: How would surveillance outcomes have changed had we sequenced less? We find that some outcomes but also certain viral lineages are more affected than others by sequencing less. However, sequencing to around a third of the original effort still captured many important outcomes for the variants of concern such as their first detection but affected more strongly other measures like the detection of first transmission clusters for some lineages. Our work highlights the importance of setting predefined targets for a national genomic surveillance program based on which sequencing effort should be determined. Additionally, the use of a centralized surveillance platform facilitates aggregating data on a national level for rapid public health responses as well as post-analyses.
Keywords: SARS-CoV-2; genomic surveillance; modeling; molecular epidemiology; platform; public health; surveillance studies; whole genome sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Oude Munnink BB, Nieuwenhuijse DF, Stein M, O’Toole Á, Haverkate M, Mollers M, Kamga SK, Schapendonk C, Pronk M, Lexmond P, et al. . 2020. Author correction: rapid SARS-CoV-2 whole-genome sequencing and analysis for informed public health decision-making in the Netherlands. Nat Med 26:1802. doi:10.1038/s41591-020-1128-5 - DOI - PMC - PubMed
-
- Chalkias S, Harper C, Vrbicky K, Walsh SR, Essink B, Brosz A, McGhee N, Tomassini JE, Chen X, Chang Y, Sutherland A, Montefiori DC, Girard B, Edwards DK, Feng J, Zhou H, Baden LR, Miller JM, Das R. 2022. A bivalent omicron-containing booster vaccine against COVID-19. N Engl J Med 387:1279–1291. doi:10.1056/NEJMoa2208343 - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

