Fascin-induced bundling protects actin filaments from disassembly by cofilin
- PMID: 38497788
- PMCID: PMC10949937
- DOI: 10.1083/jcb.202312106
Fascin-induced bundling protects actin filaments from disassembly by cofilin
Abstract
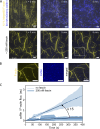
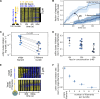
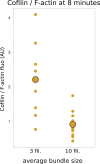
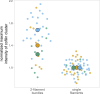
Actin filament turnover plays a central role in shaping actin networks, yet the feedback mechanism between network architecture and filament assembly dynamics remains unclear. The activity of ADF/cofilin, the main protein family responsible for filament disassembly, has been mainly studied at the single filament level. This study unveils that fascin, by crosslinking filaments into bundles, strongly slows down filament disassembly by cofilin. We show that this is due to a markedly slower initiation of the first cofilin clusters, which occurs up to 100-fold slower on large bundles compared with single filaments. In contrast, severing at cofilin cluster boundaries is unaffected by fascin bundling. After the formation of an initial cofilin cluster on a filament within a bundle, we observed the local removal of fascin. Notably, the formation of cofilin clusters on adjacent filaments is highly enhanced, locally. We propose that this interfilament cooperativity arises from the local propagation of the cofilin-induced change in helicity from one filament to the other filaments of the bundle. Overall, taking into account all the above reactions, we reveal that fascin crosslinking slows down the disassembly of actin filaments by cofilin. These findings highlight the important role played by crosslinkers in tuning actin network turnover by modulating the activity of other regulatory proteins.
© 2024 Chikireddy et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures
















Comment in
-
A step-by-step guide to fragmenting bundled actin filaments.J Cell Biol. 2024 Jun 3;223(6):e202403191. doi: 10.1083/jcb.202403191. Epub 2024 May 15. J Cell Biol. 2024. PMID: 38748453 Free PMC article.
Similar articles
-
Architecture dependence of actin filament network disassembly.Curr Biol. 2015 Jun 1;25(11):1437-47. doi: 10.1016/j.cub.2015.04.011. Epub 2015 Apr 23. Curr Biol. 2015. PMID: 25913406
-
Cofilin cooperates with fascin to disassemble filopodial actin filaments.J Cell Sci. 2011 Oct 1;124(Pt 19):3305-18. doi: 10.1242/jcs.086934. J Cell Sci. 2011. PMID: 21940796 Free PMC article.
-
Actin filament oxidation by MICAL1 suppresses protections from cofilin-induced disassembly.EMBO Rep. 2021 Feb 3;22(2):e50965. doi: 10.15252/embr.202050965. Epub 2021 Jan 4. EMBO Rep. 2021. PMID: 33393173 Free PMC article.
-
Biophysics of actin filament severing by cofilin.FEBS Lett. 2013 Apr 17;587(8):1215-9. doi: 10.1016/j.febslet.2013.01.062. Epub 2013 Feb 5. FEBS Lett. 2013. PMID: 23395798 Free PMC article. Review.
-
Tropomyosin and ADF/cofilin as collaborators and competitors.Adv Exp Med Biol. 2008;644:232-49. doi: 10.1007/978-0-387-85766-4_18. Adv Exp Med Biol. 2008. PMID: 19209826 Review.
Cited by
-
Adducin-1 Facilitates Influenza Virus Endosomal Trafficking and Uncoating by Regulating Branched Actin Dynamics and Myosin IIB Activity.Adv Sci (Weinh). 2025 Jul;12(28):e2417318. doi: 10.1002/advs.202417318. Epub 2025 Jun 5. Adv Sci (Weinh). 2025. PMID: 40472230 Free PMC article.
-
Mechanisms of actin disassembly and turnover.J Cell Biol. 2023 Dec 4;222(12):e202309021. doi: 10.1083/jcb.202309021. Epub 2023 Nov 10. J Cell Biol. 2023. PMID: 37948068 Free PMC article. Review.
-
A step-by-step guide to fragmenting bundled actin filaments.J Cell Biol. 2024 Jun 3;223(6):e202403191. doi: 10.1083/jcb.202403191. Epub 2024 May 15. J Cell Biol. 2024. PMID: 38748453 Free PMC article.
-
Reconstituted systems for studying the architecture and dynamics of actin networks.Biochem J. 2025 May 23;482(11):691-708. doi: 10.1042/BCJ20253044. Biochem J. 2025. PMID: 40411213 Free PMC article. Review.
-
A Critical Role for the Fascin Family of Actin Bundling Proteins in Axon Development, Brain Wiring and Function.bioRxiv [Preprint]. 2025 Jun 10:2025.02.21.639554. doi: 10.1101/2025.02.21.639554. bioRxiv. 2025. Update in: Mol Cell Neurosci. 2025 Sep;134:104027. doi: 10.1016/j.mcn.2025.104027. PMID: 40027761 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

