ER stress promotes mitochondrial calcium overload and activates the ROS/NLRP3 axis to mediate fatty liver ischemic injury
- PMID: 38497930
- PMCID: PMC10948136
- DOI: 10.1097/HC9.0000000000000399
ER stress promotes mitochondrial calcium overload and activates the ROS/NLRP3 axis to mediate fatty liver ischemic injury
Abstract
Background: Fatty livers are widely accepted as marginal donors for liver transplantation but are more susceptible to liver ischemia and reperfusion (IR) injury. Increased macrophage-related inflammation plays an important role in the aggravation of fatty liver IR injury. Here, we investigate the precise mechanism by which endoplasmic reticulum (ER) stress activates macrophage NOD-like receptor thermal protein domain-associated protein 3 (NLRP3) signaling by regulating mitochondrial calcium overload in fatty liver IR.
Methods: Control- and high-fat diet-fed mice were subjected to a partial liver IR model. The ER stress, mitochondrial calcium levels, and NLRP3 signaling pathway in macrophages were analyzed.
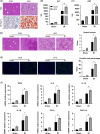
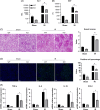
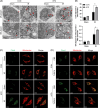
Results: Liver steatosis exacerbated liver inflammation and IR injury and enhanced NLRP3 activation in macrophages. Myeloid NLRP3 deficiency attenuated intrahepatic inflammation and fatty liver injury following IR. Mechanistically, increased ER stress and mitochondrial calcium overload were observed in macrophages obtained from mouse fatty livers after IR. Suppression of ER stress by tauroursodeoxycholic acid effectively downregulated mitochondrial calcium accumulation and suppressed NLRP3 activation in macrophages, leading to decreased inflammatory IR injury in fatty livers. Moreover, Xestospongin-C-mediated inhibition of mitochondrial calcium influx decreased reactive oxygen species (ROS) expression in macrophages after IR. Scavenging of mitochondrial ROS by mito-TEMPO suppressed macrophage NLRP3 activation and IR injury in fatty livers, indicating that excessive mitochondrial ROS production was responsible for macrophage NLRP3 activation induced by mitochondrial calcium overload. Patients with fatty liver also exhibited upregulated activation of NLRP3 and the ER stress signaling pathway after IR.
Conclusions: Our findings suggest that ER stress promotes mitochondrial calcium overload to activate ROS/NLRP3 signaling pathways within macrophages during IR-stimulated inflammatory responses associated with fatty livers.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors have no conflicts to report.
Figures





References
-
- Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology. 2020;158:1851–1864. - PubMed
-
- Gedallovich SM, Ladner DP, VanWagner LB. Liver transplantation in the era of non-alcoholic fatty liver disease/metabolic (dysfunction) associated fatty liver disease: The dilemma of the steatotic liver graft on transplantation and recipient survival. Hepatobiliary Surg Nutr. 2022;11:425–429. - PMC - PubMed
-
- Varela AT, Rolo AP, Palmeira CM. Fatty liver and ischemia/reperfusion: Are there drugs able to mitigate injury? Curr Med Chem. 2011;18:4987–5002. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

