Text vs Patient Portal Messaging to Improve Influenza Vaccination Coverage: A Health System-Wide Randomized Clinical Trial
- PMID: 38497955
- PMCID: PMC10949147
- DOI: 10.1001/jamainternmed.2024.0001
Text vs Patient Portal Messaging to Improve Influenza Vaccination Coverage: A Health System-Wide Randomized Clinical Trial
Abstract
Importance: Increasing influenza vaccination rates is a public health priority. One method recommended by the US Centers for Disease Control and Prevention and others is for health systems to send reminders nudging patients to be vaccinated.
Objective: To evaluate and compare the effect of electronic health record (EHR)-based patient portal reminders vs text message reminders on influenza vaccination rates across a health system.
Design, setting, and participants: This 3-arm randomized clinical trial was conducted from September 7, 2022, to April 30, 2023, among primary care patients within the University of California, Los Angeles (UCLA) health system.
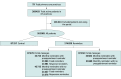
Interventions: Arm 1 received standard of care. The health system sent monthly reminder messages to patients due for an influenza vaccine by portal (arm 2) or text (arm 3). Arm 2 had a 2 × 2 nested design, with fixed vs responsive monthly reminders and preappointment vs no preappointment reminders. Arm 3 had 1 × 2 design, with preappointment vs no preappointment reminders. Preappointment reminders for eligible patients were sent 24 and 48 hours before scheduled primary care visits. Fixed reminders (in October, November, and December) involved identical messages via portal or text. Responsive portal reminders involved a September message asking patients about their plans for vaccination, with a follow-up reminder if the response was affirmative but the patient was not yet vaccinated.
Main outcomes and measures: The primary outcome was influenza vaccination by April 30, 2023, obtained from the UCLA EHR, including vaccination from pharmacies and other sources.
Results: A total of 262 085 patients (mean [SD] age, 45.1 [20.7] years; 237 404 [90.6%] adults; 24 681 [9.4%] children; 149 349 [57.0%] women) in 79 primary care practices were included (87 257 in arm 1, 87 478 in arm 2, and 87 350 in arm 3). At the entire primary care population level, none of the interventions improved influenza vaccination rates. All groups had rates of approximately 47%. There was no statistical or clinically significant improvement following portal vs text, preappointment reminders vs no preappointment reminders (portal and text reminders combined), or responsive vs fixed monthly portal reminders.
Conclusions and relevance: At the population level, neither portal nor text reminders for influenza vaccination were effective. Given that vaccine hesitancy may be a major reason for the lack of impact of portal or text reminders, more intensive interventions by health systems are needed to raise influenza vaccination coverage levels.
Trial registration: ClinicalTrials.gov Identifier: NCT05525494.
Conflict of interest statement
References
-
- US Centers for Disease Control and Prevention . 2022-2023 US flu season: preliminary in-season burden estimates. Accessed June 14, 2023. https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm
-
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices–United States, 2018-19 influenza season. MMWR Recomm Rep. 2018;67(3):1-20. doi: 10.15585/mmwr.rr6703a1 - DOI - PMC - PubMed
-
- US Centers for Disease Control and Prevention . Flu vaccination coverage, United States, 2021–22 influenza season. Accessed June 14, 2023. https://www.cdc.gov/flu/fluvaxview/coverage-2022estimates.htm
-
- US Centers for Disease Control and Prevention . Weekly flu vaccination dashboard. Accessed June 14, 2023. https://www.cdc.gov/flu/fluvaxview/dashboard/vaccination-dashboard.html