Magnetic Resonance Imaging Characteristics of LGI1-Antibody and CASPR2-Antibody Encephalitis
- PMID: 38497971
- PMCID: PMC10949153
- DOI: 10.1001/jamaneurol.2024.0126
Magnetic Resonance Imaging Characteristics of LGI1-Antibody and CASPR2-Antibody Encephalitis
Abstract
Importance: Rapid and accurate diagnosis of autoimmune encephalitis encourages prompt initiation of immunotherapy toward improved patient outcomes. However, clinical features alone may not sufficiently narrow the differential diagnosis, and awaiting autoantibody results can delay immunotherapy.
Objective: To identify simple magnetic resonance imaging (MRI) characteristics that accurately distinguish 2 common forms of autoimmune encephalitis, LGI1- and CASPR2-antibody encephalitis (LGI1/CASPR2-Ab-E), from 2 major differential diagnoses, viral encephalitis (VE) and Creutzfeldt-Jakob disease (CJD).
Design, setting, and participants: This cross-sectional study involved a retrospective, blinded analysis of the first available brain MRIs (taken 2000-2022) from 192 patients at Oxford University Hospitals in the UK and Mayo Clinic in the US. These patients had LGI1/CASPR2-Ab-E, VE, or CJD as evaluated by 2 neuroradiologists (discovery cohort; n = 87); findings were validated in an independent cohort by 3 neurologists (n = 105). Groups were statistically compared with contingency tables. Data were analyzed in 2023.
Main outcomes and measures: MRI findings including T2 or fluid-attenuated inversion recovery (FLAIR) hyperintensities, swelling or volume loss, presence of gadolinium contrast enhancement, and diffusion-weighted imaging changes. Correlations with clinical features.
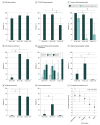
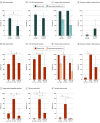
Results: Among 192 participants with MRIs reviewed, 71 were female (37%) and 121 were male (63%); the median age was 66 years (range, 19-92 years). By comparison with VE and CJD, in LGI1/CASPR2-Ab-E, T2 and/or FLAIR hyperintensities were less likely to extend outside the temporal lobe (3/42 patients [7%] vs 17/18 patients [94%] with VE; P < .001, and 3/4 patients [75%] with CJD; P = .005), less frequently exhibited swelling (12/55 [22%] with LGI1/CASPR2-Ab-E vs 13/22 [59%] with VE; P = .003), and showed no diffusion restriction (0 patients vs 16/22 [73%] with VE and 8/10 [80%] with CJD; both P < .001) and rare contrast enhancement (1/20 [5%] vs 7/17 [41%] with VE; P = .01). These findings were validated in an independent cohort and generated an area under the curve of 0.97, sensitivity of 90%, and specificity of 95% among cases with T2/FLAIR hyperintensity in the hippocampus and/or amygdala.
Conclusions and relevance: In this study, T2 and/or FLAIR hyperintensities confined to the temporal lobes, without diffusion restriction or contrast enhancement, robustly distinguished LGI1/CASPR2-Ab-E from key differential diagnoses. These observations should assist clinical decision-making toward expediting immunotherapy. Their generalizability to other forms of autoimmune encephalitis and VE should be examined in future studies.
Conflict of interest statement
Figures


References
-
- Irani SR, Alexander S, Waters P, et al. . Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133(9):2734-2748. doi:10.1093/brain/awq213 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

