The TLK-ASF1 histone chaperone pathway plays a critical role in IL-1β-mediated AML progression
- PMID: 38498025
- PMCID: PMC11340594
- DOI: 10.1182/blood.2023022079
The TLK-ASF1 histone chaperone pathway plays a critical role in IL-1β-mediated AML progression
Abstract
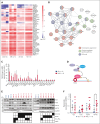
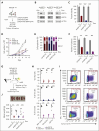
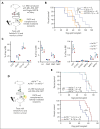
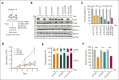
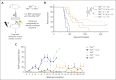
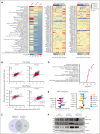
Identifying and targeting microenvironment-driven pathways that are active across acute myeloid leukemia (AML) genetic subtypes should allow the development of more broadly effective therapies. The proinflammatory cytokine interleukin-1β (IL-1β) is abundant in the AML microenvironment and promotes leukemic growth. Through RNA-sequencing analysis, we identify that IL-1β-upregulated ASF1B (antisilencing function-1B), a histone chaperone, in AML progenitors compared with healthy progenitors. ASF1B, along with its paralogous protein ASF1A, recruits H3-H4 histones onto the replication fork during S-phase, a process regulated by Tousled-like kinase 1 and 2 (TLKs). Although ASF1s and TLKs are known to be overexpressed in multiple solid tumors and associated with poor prognosis, their functional roles in hematopoiesis and inflammation-driven leukemia remain unexplored. In this study, we identify that ASF1s and TLKs are overexpressed in multiple genetic subtypes of AML. We demonstrate that depletion of ASF1s significantly reduces leukemic cell growth in both in vitro and in vivo models using human cells. Using a murine model, we show that overexpression of ASF1B accelerates leukemia progression. Moreover, Asf1b or Tlk2 deletion delayed leukemia progression, whereas these proteins are dispensable for normal hematopoiesis. Through proteomics and phosphoproteomics analyses, we uncover that the TLK-ASF1 pathway promotes leukemogenesis by affecting the cell cycle and DNA damage pathways. Collectively, our findings identify the TLK1-ASF1 pathway as a novel mediator of inflammatory signaling and a promising therapeutic target for AML treatment across diverse genetic subtypes. Selective inhibition of this pathway offers potential opportunities to intervene effectively, address intratumoral heterogeneity, and ultimately improve clinical outcomes in AML.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–1907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

