Preliminary Efficacy of a Cognitive Behavioral Therapy-Based Smartphone App for Smoking Cessation in China: Randomized Controlled Pilot Trial
- PMID: 38498030
- PMCID: PMC10985609
- DOI: 10.2196/48050
Preliminary Efficacy of a Cognitive Behavioral Therapy-Based Smartphone App for Smoking Cessation in China: Randomized Controlled Pilot Trial
Abstract
Background: The overall prevalence of cigarette smokers in China is very high, and China's total cigarette consumption makes up more than 40% of the world's consumption. In view of the lack of smoking cessation services and social support in China and the effectiveness of mobile phone apps for quitting smoking in other countries, we carried out a smartphone app-based smoking cessation trial in China.
Objective: This study aimed to evaluate the efficacy of a cognitive behavioral therapy (CBT)-based smoking cessation smartphone app among smokers seeking treatment in China.
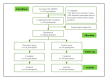
Methods: We conducted a randomized controlled, web-based pilot clinical trial in China between February 23 and June 27, 2021. Eligible participants were randomly assigned to the smoking cessation app intervention group or the control group in a ratio of 1:1. The intervention group received the CBT smoking cessation intervention using a smartphone app, and the control group received a "thank you" message. The intervention was 4 weeks long, and the patients were followed up for 4 weeks. The primary outcome was self-reported continuous smoking abstinence at week 4 after the quit date. The secondary outcomes included self-reported 7-day point prevalence of smoking abstinence; reduction of the number of cigarettes smoked per day at weeks 1, 2, 3, and 4; and program acceptability.
Results: A total of 973 people were recruited to quit smoking, of whom 262 completed basic information, 56 were excluded, and 206 were randomized and included in the final analysis. There were 189 (91.7%) men and 17 (8.3%) women, with an average age of 34.46 (SD 7.53) years and an average daily smoking rate of 15.93 (SD 7.10) cigarettes/day. We found 30 (29.7%) of the 101 participants in the intervention group and 7 (6.7%) of the 105 participants in the control group reported continuous smoking cessation after the quit date at week 4 (odds ratio 5.92, 95% CI 3.78-9.26; P<.001). The 7-day point prevalence abstinence rate of the intervention group varied from 42.6% (43/101) to 46.5% (47/101) after 1, 2, 3, and 4 weeks, while the control group varied from 18.1% (19/105) to 26.7% (28/105). Compared to the control group, continued smokers consumed 1.5-3.0 fewer cigarettes per day in the intervention group. The overall program got positive user feedback with a high satisfaction rate (66/87, 76%) and an average Mobile Application Rating Scale user version score of 3.46.
Conclusions: Our pilot study provided preliminary evidence that the CBT-based smoking cessation smartphone app led to improved smoking quit rates versus control in Chinese smokers. The study demonstrated the CBT-based smartphone app may be an effective and feasible digital treatment model to help smokers quit, which may improve smoking cessation service quality and accessibility in China.
Trial registration: ClinicalTrials.gov NCT04421170; https://clinicaltrials.gov/study/NCT04421170.
International registered report identifier (irrid): RR2-10.1136/bmjopen-2020-041985.
Keywords: China; cognitive behavioral therapy; program acceptability; randomized controlled trial; smartphone app; smoking cessation.
©Shanshan Chen, Jinsong Tang, Congyang Wu, Ge Zhang, Jing Zhang, Yanhui Liao. Originally published in JMIR Formative Research (https://formative.jmir.org), 18.03.2024.
Conflict of interest statement
Conflicts of Interest: YL received funding from Johnson & Johnson Pharmaceutical Company for the study. SC, JT, CW, GZ, JZ, and YL have no potential conflicts of interest to declare.
References
-
- Chu KH, Matheny SJ, Escobar-Viera CG, Wessel C, Notier AE, Davis EM. Smartphone health apps for tobacco cessation: a systematic review. Addict Behav. 2021;112:106616. doi: 10.1016/j.addbeh.2020.106616. https://europepmc.org/abstract/MED/32932102 S0306-4603(20)30746-2 - DOI - PMC - PubMed
-
- Tobacco. World Health Organization. 2023. [2023-12-07]. https://www.who.int/news-room/fact-sheets/detail/tobacco .
-
- Wang M, Luo X, Xu S, Liu W, Ding F, Zhang X, Wang L, Liu J, Hu J, Wang W. Trends in smoking prevalence and implication for chronic diseases in China: serial national cross-sectional surveys from 2003 to 2013. Lancet Respir Med. 2019;7(1):35–45. doi: 10.1016/S2213-2600(18)30432-6.S2213-2600(18)30432-6 - DOI - PubMed
-
- Eriksen MM, Ross H. The tobacco atlas (fourth edition) American Cancer Society and World Lung Foundation. 2014. [2014-10-07]. http://www.tobaccoatlas.org/
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous