Investigating Nonspecific Effects of the Live-Attenuated Japanese Encephalitis Vaccine on Lower Respiratory Tract Infections in Children Aged 25-35 Months: Retrospective Cohort Study
- PMID: 38498052
- PMCID: PMC10993859
- DOI: 10.2196/53040
Investigating Nonspecific Effects of the Live-Attenuated Japanese Encephalitis Vaccine on Lower Respiratory Tract Infections in Children Aged 25-35 Months: Retrospective Cohort Study
Abstract
Background: Live attenuated vaccines may be used to prevent nontargeted diseases such as lower respiratory tract infections (LRTIs) due to their nonspecific effects (NSEs).
Objective: We aimed to analyze the NSEs of the Japanese encephalitis vaccine on pediatric LRTIs in children aged 25 months to 35 months.
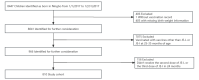
Methods: A retrospective cohort study was conducted by using a population-based electronic health record database in Zhejiang, China. Enrolled participants were children born from January 1, 2017, to December 31, 2017, and who were inoculated with the live-attenuated Japanese encephalitis vaccine (JE-L) or inactivated Japanese encephalitis vaccine (JE-I) as the most recent vaccine at 24 months of age. The study was carried out between January 1, 2019, and December 31, 2019. All inpatient and outpatient hospital visits for LRTIs among children aged 25 months to 35 months were recorded. The Andersen-Gill model was used to assess the NSEs of JE-L against LRTIs in children and compared with those of JE-I as the most recent vaccine.
Results: A total of 810 children born in 2017 were enrolled, of whom 585 received JE-L (JE-L cohort) and 225 received JE-I (JE-I cohort) as their last vaccine. The JE-L cohort showed a reduced risk of LRTIs (adjusted hazard ratio [aHR] 0.537, 95% CI 0.416-0.693), including pneumonia (aHR 0.501, 95% CI 0.393-0.638) and acute bronchitis (aHR 0.525, 95% CI 0.396-0.698) at 25 months to 35 months of age. The NSEs provided by JE-L were especially pronounced in female children (aHR 0.305, 95% CI 0.198-0.469) and children without chronic diseases (aHR 0.553, 95% CI 0.420-0.729), without siblings (aHR 0.361, 95% CI 0.255-0.511), with more than 30 inpatient and outpatient hospital visits prior to 24 months of age (aHR 0.163, 95% CI 0.091-0.290), or with 5 to 10 inpatient and outpatient hospital visits due to infectious diseases prior to 24 months old (aHR 0.058, 95% CI 0.017-0.202).
Conclusions: Compared with JE-I, receiving JE-L as the most recent vaccine was associated with lower risk of inpatient and outpatient hospital visits for LRTIs among children aged 25 months to 35 months. The nature of NSEs induced by JE-L should be considered for policymakers and physicians when recommending JE vaccines to those at high risk of infection from the Japanese encephalitis virus.
Keywords: Anderson Gill model; Japanese encephalitis vaccine; lower respiratory tract infectious diseases; nonspecific effect of vaccines; trained immunity.
©Siyi Zhan, Hongbo Lin, Yingying Yang, Tao Chen, Sheng Mao, Chuanxi Fu. Originally published in JMIR Public Health and Surveillance (https://publichealth.jmir.org), 18.03.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Koppaka R, Global Public Health Achievements Team Ten great public health achievements --- worldwide, 2001--2010. Morbidity and Mortality Weekly Report (MMWR) 2011;60(24):814–8. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6024a4.htm - PubMed
-
- Biering-Sørensen S, Aaby P, Lund N, Monteiro I, Jensen KJ, Eriksen HB, Schaltz-Buchholzer F, Jørgensen ASP, Rodrigues A, Fisker AB, Benn CS. Early BCG-Denmark and neonatal mortality among infants weighing less than 2500 g: a randomized controlled trial. Clin Infect Dis. 2017 Oct 01;65(7):1183–1190. doi: 10.1093/cid/cix525. https://europepmc.org/abstract/MED/29579158 4079383 - DOI - PMC - PubMed
-
- Sørup S, Stensballe LG, Krause TG, Aaby P, Benn CS, Ravn H. Oral polio vaccination and hospital admissions with non-polio infections in Denmark: nationwide retrospective cohort study. Open Forum Infect Dis. 2016 Jan;3(1):ofv204. doi: 10.1093/ofid/ofv204. https://europepmc.org/abstract/MED/26885538 ofv204 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical