Long-Term Efficacy Following Intra-articular Injection of Carboxymethyl-chitosan, a New Product Class for Knee Osteoarthritis: Results from an Observational Study in Germany
- PMID: 38498142
- PMCID: PMC11111638
- DOI: 10.1007/s40744-024-00661-6
Long-Term Efficacy Following Intra-articular Injection of Carboxymethyl-chitosan, a New Product Class for Knee Osteoarthritis: Results from an Observational Study in Germany
Abstract
Introduction: Evaluate the real-world efficacy of a single intra-articular injection of carboxymethyl-chitosan (CM-chitosan), a new product class for knee osteoarthritis (OA).
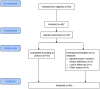
Methods: This post-marketing study included adult patients with knee OA, who received a single injection of 60 mg CM-chitosan (currently marketed as KioMedinevsone) according to the instructions for use. Follow-up was performed at weeks 1, 12, 24, and 36. Efficacy was evaluated using a Visual Analog Scale (VAS) score for pain, the Knee injury and Osteoarthritis Outcome Score (KOOS), Patient's Global Assessment (PGA), and overall patient satisfaction.
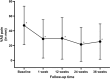
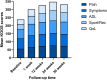
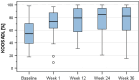
Results: Forty-nine patients were included in the study. VAS pain score significantly decreased from a median of 49.0 mm at baseline to 24.0 mm at week 1 and to 18 mm at week 36. Pain improvement was stable since at week 36; 91.8% of patients confirmed pain reduction. All KOOS subscales (symptoms, pain, activities of daily living, sports and recreational activities, quality of life) improved significantly compared to baseline at all time points. KOOS pain improved progressively from a median of 58.3% at baseline (mean 56.2 ± 18.8%) to 86.1% (mean 74.1 ± 24%) at week 36 compared to baseline. Overall, more than 70% of patients reported a condition gain (PGA), matching well with the more than 75% of patients being satisfied with the treatment. At 6 months, 72.7% of the patients could be classified as responder according to the OMERACT-OARSI proposed set of responder criteria.
Conclusion: CM-chitosan showed a rapid onset of pain relief after 1 week and with a duration of 9 months. In a real-world setting, treatment with CM-chitosan would appear to be a potentially effective option to reduce pain and improve physical function and global condition in patients with knee OA, opening new perspectives in patients who are considered as refractory to current symptomatic therapies and where the unmet need is high.
Trial registration number: NCT04757051 (ClinicalTrials.gov).
Keywords: CM-chitosan; Joint; Knee; Osteoarthritis; Real-life setting; Real-world evidence.
© 2024. The Author(s).
Conflict of interest statement
Nicolas Portelange, Mickael Chausson and Wim Weyenberg are employees for KiOmed Pharma, Herstal, Belgium. Nils Lynen and Christoph Eichhorn have nothing to disclose.
Figures

References
-
- Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29–30:100587. https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(20)30331.... Accessed 07 Aug 2023. - PMC - PubMed
-
- Wittenauer R, Smith L, Aden K. Background paper 612 osteoarthritis. Geneva: World Health Organisation; 2013.
Associated data
LinkOut - more resources
Full Text Sources
Medical

