Granzyme B degrades extracellular matrix and promotes inflammation and choroidal neovascularization
- PMID: 38498232
- PMCID: PMC11303490
- DOI: 10.1007/s10456-024-09909-9
Granzyme B degrades extracellular matrix and promotes inflammation and choroidal neovascularization
Erratum in
-
Correction: Granzyme B degrades extracellular matrix and promotes inflammation and choroidal neovascularization.Angiogenesis. 2024 Aug;27(3):375-377. doi: 10.1007/s10456-024-09919-7. Angiogenesis. 2024. PMID: 38700585 Free PMC article. No abstract available.
Abstract
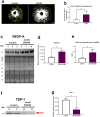
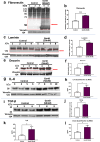
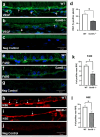
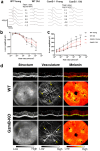
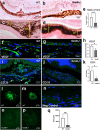
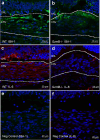
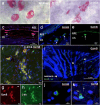
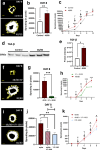
Age-related macular degeneration (AMD) is a common retinal neurodegenerative disease among the elderly. Neovascular AMD (nAMD), a leading cause of AMD-related blindness, involves choroidal neovascularization (CNV), which can be suppressed by anti-angiogenic treatments. However, current CNV treatments do not work in all nAMD patients. Here we investigate a novel target for AMD. Granzyme B (GzmB) is a serine protease that promotes aging, chronic inflammation and vascular permeability through the degradation of the extracellular matrix (ECM) and tight junctions. Extracellular GzmB is increased in retina pigment epithelium (RPE) and mast cells in the choroid of the healthy aging outer retina. It is further increased in donor eyes exhibiting features of nAMD and CNV. Here, we show in RPE-choroidal explant cultures that exogenous GzmB degrades the RPE-choroid ECM, promotes retinal/choroidal inflammation and angiogenesis while diminishing anti-angiogenic factor, thrombospondin-1 (TSP-1). The pharmacological inhibition of either GzmB or mast-cell degranulation significantly reduces choroidal angiogenesis. In line with our in vitro data, GzmB-deficiency reduces the extent of laser-induced CNV lesions and the age-related deterioration of electroretinogram (ERG) responses in mice. These findings suggest that targeting GzmB, a serine protease with no known endogenous inhibitors, may be a potential novel therapeutic approach to suppress CNV in nAMD.
Keywords: Age-related macular degeneration; Angiogenesis; Choroidal neovascularization; Extracellular matrix; Granzyme B; Inflammation; Mast cell.
© 2024. The Author(s).
Conflict of interest statement
D.J.G. is co-founder and chief scientific officer of viDA Therapeutics. We received VTI-1002 as a generous gift. The remaining authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

