Deciphering Physio-Biochemical Basis of Tolerance Mechanism for Sesame (Sesamum indicum L.) Genotypes under Waterlogging Stress at Early Vegetative Stage
- PMID: 38498414
- PMCID: PMC10892085
- DOI: 10.3390/plants13040501
Deciphering Physio-Biochemical Basis of Tolerance Mechanism for Sesame (Sesamum indicum L.) Genotypes under Waterlogging Stress at Early Vegetative Stage
Abstract
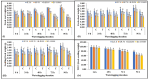
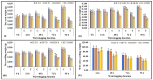
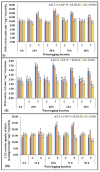
Waterlogging represents a substantial agricultural concern, inducing harmful impacts on crop development and productivity. In the present study, 142 diverse sesame genotypes were examined during the early vegetative phase to assess their response under waterlogging conditions. Based on the severity of symptoms observed, 2 genotypes were classified as highly tolerant, 66 as moderately tolerant, 69 as susceptible, and 5 as highly susceptible. Subsequent investigation focused on four genotypes, i.e., two highly tolerant (JLT-8 and GP-70) and two highly susceptible (R-III-F6 and EC-335003). These genotypes were subjected to incremental stress periods (0 h, 24 h, 48 h, 72 h, and 96 h) to elucidate the biochemical basis of tolerance mechanisms. Each experiment was conducted as a randomized split-plot design with three replications, and the statistical significance of the treatment differences was determined using the one-way analysis of variance (ANOVA) followed by the Fisher least significant difference (LSD) test at p ≤ 0.05. The influence of waterlogging stress on morphological growth was detrimental for both tolerant and susceptible genotypes, with more severe consequences observed in the latter. Although adventitious roots were observed in both sets of genotypes above flooding levels, the tolerant genotypes exhibited a more rapid and vigorous development of these roots after 48 h of stress exposure. Tolerant genotypes displayed higher tolerance coefficients compared to susceptible genotypes. Furthermore, tolerant genotypes maintained elevated antioxidant potential, thereby minimizing oxidative stress. Conversely, susceptible genotypes exhibited higher accumulation of hydrogen peroxide (H2O2) and malondialdehyde content. Photosynthetic efficiency was reduced in all genotypes after 24 h of stress treatment, with a particularly drastic reduction in susceptible genotypes compared to their tolerant counterparts. Tolerant genotypes exhibited significantly higher activities of anaerobic metabolism enzymes, enabling prolonged survival under waterlogging conditions. Increase in proline content was observed in all the genotypes indicating the cellular osmotic balance adjustments in response to stress exposure. Consequently, the robust antioxidant potential and efficient anaerobic metabolism observed in the tolerant genotypes served as key mechanisms enabling their resilience to short-term waterlogging exposure. These findings underscore the promising potential of specific sesame genotypes in enhancing crop resilience against waterlogging stress, offering valuable insights for agricultural practices and breeding programs.
Keywords: antioxidant enzymes; ethanolic fermentation; reactive oxygen species; sesame; waterlogging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Liu K., Harrison M.T., Yan H., Liu D.L., Meinke H., Hoogenboom G., Wang B., Peng B., Guan K., Jaegermeyr J., et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023;14:765. doi: 10.1038/s41467-023-36129-4. - DOI - PMC - PubMed
-
- Vartapetian B.B., Jackson M.B. Plant Adaptations to Anaerobic Stress. Ann. Bot. 1997;79:3–20. doi: 10.1093/oxfordjournals.aob.a010303. - DOI
-
- Chugh V., Gupta A.K., Grewal M.S., Kaur N. Response of Antioxidative and Ethanolic Fermentation Enzymes in Maize Seedlings of Tolerant and Sensitive Genotypes under Short-Term Waterlogging. Indian J. Exp. Biol. 2012;50:577–582. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

