CAR19 monitoring by peripheral blood immunophenotyping reveals histology-specific expansion and toxicity
- PMID: 38498731
- PMCID: PMC11258628
- DOI: 10.1182/bloodadvances.2024012637
CAR19 monitoring by peripheral blood immunophenotyping reveals histology-specific expansion and toxicity
Abstract
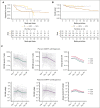
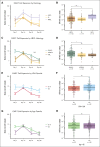
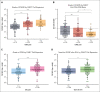
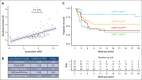
Chimeric antigen receptor (CAR) T cells directed against CD19 (CAR19) are a revolutionary treatment for B-cell lymphomas (BCLs). CAR19 cell expansion is necessary for CAR19 function but is also associated with toxicity. To define the impact of CAR19 expansion on patient outcomes, we prospectively followed a cohort of 236 patients treated with CAR19 (brexucabtagene autoleucel or axicabtagene ciloleucel) for mantle cell lymphoma (MCL), follicular lymphoma, and large BCL (LBCL) over the course of 5 years and obtained CAR19 expansion data using peripheral blood immunophenotyping for 188 of these patients. CAR19 expansion was higher in patients with MCL than other lymphoma histologic subtypes. Notably, patients with MCL had increased toxicity and required fourfold higher cumulative steroid doses than patients with LBCL. CAR19 expansion was associated with the development of cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and the requirement for granulocyte colony-stimulating factor 14 days after infusion. Younger patients and those with elevated lactate dehydrogenase (LDH) had significantly higher CAR19 expansion. In general, no association between CAR19 expansion and LBCL treatment response was observed. However, when controlling for tumor burden, we found that lower CAR19 expansion in conjunction with low LDH was associated with improved outcomes in LBCL. In sum, this study finds CAR19 expansion principally associates with CAR-related toxicity. Additionally, CAR19 expansion as measured by peripheral blood immunophenotyping may be dispensable to favorable outcomes in LBCL.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.P.H. served on an advisory board for Kite Pharmaceuticals. Z.G. is an inventor on 2 patent applications; holds equity in Boom Capital Ventures; and is a consultant for Mubadala Ventures. S.B. reports consulting for Allogene. C.L.M. is a founder, equity holder, consultant, and director of Cargo Therapeutics and Link Cell Therapies; reports equity in Lyell Immunopharma and royalties from the National Institutes of Health for CAR22; and consults for Immatics, Ensoma, Mammoth, Adaptimmune, and Bristol Myers Squibb. L.M. reports research support from Adaptive Biotechnologies and Servier Laboratories, and consults for Amgen and Pfizer. P.S. reports research support from Kite Pharma-Gilead. S. Sidana reports consulting for Janssen. D.M.K. reports consultancy for Roche, Adaptive Biotechnologies, and Genentech, and equity ownership interest in Foresight Diagnostics. A.A.A. reports consulting for and having stock options in CiberMed; consulting fees and research funding from Celgene; holding stock options in CAPP Medical; consulting fees, honoraria, and travel funding from Roche; holding stop options in Forty Seven; holding stock options in Syncopation Life Sciences; consulting fees and travel fund from Gilead; consulting fees from and holding stock options in Foresight Diagnostics; honoraria from Jannsen; and consulting for Lymphoma research foundation. M.J.F. reports consulting for Kite Pharma-Gilead, Adaptative Biotechnologies, and Cargo Therapeutics, and research support from Kite Pharma-Gilead, Allogene Therapeutics, Cargo Therapeutics, and Adaptative Biotechnologies. D.B.M. reports consulting for Kite Pharma-Gilead, Juno Therapeutics-Celgene, Novartis, Janssen, and Pharmacyclics, and research support from Kite Pharma-Gilead, Allogene Therapeutics, Cargo Therapeutics, Pharmacyclics, Miltenyi Biotec, and Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Figures


Comment in
-
CAR T-cell expansion: harmful or helpful?Blood Adv. 2024 Jun 25;8(12):3311-3313. doi: 10.1182/bloodadvances.2024013146. Blood Adv. 2024. PMID: 38916899 Free PMC article. No abstract available.
References
-
- Locke FL, Miklos DB, Jacobson CA, et al. All ZUMA-7 Investigators and Contributing Kite Members Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

