EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update
- PMID: 38499325
- PMCID: PMC11103320
- DOI: 10.1136/ard-2024-225531
EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update
Abstract
Objective: New modes of action and more data on the efficacy and safety of existing drugs in psoriatic arthritis (PsA) required an update of the EULAR 2019 recommendations for the pharmacological treatment of PsA.
Methods: Following EULAR standardised operating procedures, the process included a systematic literature review and a consensus meeting of 36 international experts in April 2023. Levels of evidence and grades of recommendations were determined.
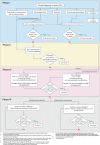
Results: The updated recommendations comprise 7 overarching principles and 11 recommendations, and provide a treatment strategy for pharmacological therapies. Non-steroidal anti-inflammatory drugs should be used in monotherapy only for mild PsA and in the short term; oral glucocorticoids are not recommended. In patients with peripheral arthritis, rapid initiation of conventional synthetic disease-modifying antirheumatic drugs is recommended and methotrexate preferred. If the treatment target is not achieved with this strategy, a biological disease-modifying antirheumatic drug (bDMARD) should be initiated, without preference among modes of action. Relevant skin psoriasis should orient towards bDMARDs targeting interleukin (IL)-23p40, IL-23p19, IL-17A and IL-17A/F inhibitors. In case of predominant axial or entheseal disease, an algorithm is also proposed. Use of Janus kinase inhibitors is proposed primarily after bDMARD failure, taking relevant risk factors into account, or in case bDMARDs are not an appropriate choice. Inflammatory bowel disease and uveitis, if present, should influence drug choices, with monoclonal tumour necrosis factor inhibitors proposed. Drug switches and tapering in sustained remission are also addressed.
Conclusion: These updated recommendations integrate all currently available drugs in a practical and progressive approach, which will be helpful in the pharmacological management of PsA.
Keywords: Biological Therapy; Biosimilar Pharmaceuticals; Psoriatic Arthritis; Treatment.
© European Alliance of Associations for Rheumatology, EULAR 2024. Re-use permitted under CC BY-NC-ND. No commercial re-use. No derivatives. See rights and permissions. Published by BMJ on behalf of EULAR.
Conflict of interest statement
Competing interests: No support to any author for the present work. Outside the submitted work: LG: research grants: AbbVie, Biogen, Lilly, Novartis, UCB; consulting fees: AbbVie, Amgen, BMS, Celltrion, Janssen, Lilly, MSD, Novartis, Pfizer, UCB; non-financial support: AbbVie, Amgen, Galapagos, Janssen, MSD, Novartis, Pfizer, UCB; membership on an entity’s Board of Directors or advisory committees: EULAR Treasurer. AK: speakers bureau, consultancy: AbbVie, Amgen, Galapagos, Janssen, Eli Lilly, MSD, Novartis, Pfizer, UCB. RJOF: research grants: Medac, Lilly; consulting fees: Sanofi. DA: research grants: Galapagos, Lilly; consulting fees: AbbVie, Gilead, Janssen, Lilly, Merck, Novartis, Sanofi. XB: research grants: AbbVie, MSD, Novartis; consultancies: AbbVie, Amgen, Celltrion, Chugai, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, Roche, Sandoz, UCB; membership on an entity’s Board of Directors or advisory committees: ASAS President, EULAR President Elect. W-HB: honoraria: AbbVie, Almirall, BMS, Janssen, Leo, Eli Lilly, Novartis, UCB; expert testimony: Novartis; participation on a Data Safety Monitoring Board or Advisory Board: AbbVie, Almirall, BMS, Janssen, Leo, Eli Lilly, Novartis, UCB. IBM: honoraria/consultation fees non-exec roles: NHS GGC Board Member, Evelo Board of Directors, Versus Arthritis Trustee Status; stock or stock options: Evelo, Cabaletta, Compugen, Causeway Therapeutics, Dextera. DGM: research grants: Janssen, AbbVie, Lilly, Novartis, UCB, BMS, Moonlake; consulting fees: Janssen, AbbVie, Lilly, Novartis, UCB, BMS, Moonlake, Celgene; honoraria: Janssen, AbbVie, Lilly, Novartis, UCB, BMS, Moonlake. KLW: research grants: BMS, Pfizer; consulting: Pfizer, AbbVie, AstraZeneca, BMS, Eli Lilly, Galapagos, GlaxoSmithKline (GSK), Gilead, Novartis, Moderna, Regeneron, Roche, Sanofi, UCB Pharma. AB: speakers fees: AbbVie, Amgen, AlphaSigma, AstraZeneca, Angelini, Biogen, BMS, Berlin-Chemie, Boehringer Ingelheim, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Teva, UCB, Zentiva; consultancies: Akros, AbbVie, Amgen, AlphaSigma, Biogen, Boehringer Ingelheim, Lilly, Mylan, MSD, Novartis, Pfizer, Roche, Sandoz, Sobi, UCB. PVB: consulting fees: AbbVie, Janssen-Cilag, Pfizer; honoraria: AbbVie, Bausch Health, Celltrion Healthcare, Eli Lilly, Gedeon Richter, IBSA Pharma, Infomed, Janssen-Cilag, Novartis, Pfizer, Sandoz; payment for expert testimony: Gedeon Richter; other: President, Hungarian Association of Rheumatologists. GRB: honoraria and/or speaker fees: AbbVie, BMS, Janssen, Lilly, Novartis, Pfizer. JDC: honoraria: UCB. PC: research grants: AbbVie, Amgen, Biogen, Jansen, Lilly, Novartis, UCB; consulting fees: AbbVie, Amgen, Celltrion, Janssen, Lilly, MSD, Novartis, Pfizer, UCB. LE: consultation fee/advisory board: AbbVie, Novartis, Janssen, UCB, BMS, Eli Lilly; research/educational grants: AbbVie, Fresenius Kabi, Janssen, Amgen, UCB, Novartis, Eli Lilly, Sandoz, Pfizer. MLH: grant support: AbbVie, Biogen, BMS, Celltrion, Eli Lilly, Janssen Biologics BV, Lundbeck Foundation, MSD, Pfizer, Roche, Samsung Bioepis, Sandoz, Novartis, Nordforsk; honoraria: Pfizer, Medac, Sandoz; advisory board: AbbVie; past-chair of the steering committee of the Danish Rheumatology Quality Registry (DANBIO, DRQ), which receives public funding from the hospital owners and funding from pharmaceutical companies; cochair of EuroSpA, partly funded by Novartis. AI: research grants from AbbVie, Pfizer, Novartis; honoraria for lectures, presentations, speakers bureaus from AbbVie, Alfasigma, BMS, Celgene, Celltrion, Eli Lilly, Galapagos, Gilead, Janssen, MSD, Novartis, Pfizer, Sanofi Genzyme, Sobi; EULAR Board Member; EULAR Congress Committee, Education Committee and Advocacy Committee Advisor; EULAR Past President. LEK: consultancies: AbbVie, Amgen, Biogen, BMS, Celgene, Eli Lilly, Pfizer, UCB, Sanofi, GSK, Galapagos, Forward Pharma, MSD, Novartis, Janssen; has been representing rheumatology FOREUM scientific chair. RQ: consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, Amgen-Celgene, Eli Lilly, Novartis, Janssen, Pfizer, MSD, UCB. DM: honoraria: UCB, Janssen, GSK, AstraZeneca, AbbVie; support to meetings: Janssen. HM-O: grant support: Janssen, Novartis, UCB; honoraria and/or speaker fees: AbbVie, Biogen, Eli Lilly, Janssen, Moonlake, Novartis, Pfizer, Takeda, UCB. PJM: grant support: AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Eli Lilly, Genascence, Janssen, Novartis, Pfizer, UCB; consulting fees: AbbVie, Acelyrin, Aclaris, Alumis, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Genascence, Inmagene, Janssen, Moonlake, Novartis, Pfizer, Takeda, UCB, Ventyx, Xinthera; honoraria: AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, UCB. PN: consulting fees and honoraria: AbbVie, Amgen, BMS, Lilly, Janssen, GSK, Novartis, UCB, Servatus; boards: Amgen, BMS, Janssen, GSK, Novartis, UCB; GRAPPA Steering Committee, Chair ASMPOC, ARA. LS: consulting fees: AbbVie, Almirall, Novartis, Janssen, Lilly, UCB, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim; honoraria: AbbVie, Almirall, Novartis, Janssen, UCB, Pfizer, Takeda, Galderma, Biogen, Celgene, Celltrion, Lilly, Sanofi, Bristol Myers Squibb, Boehringer Ingelheim; support to attending meetings: AbbVie, Janssen, Lilly, Novartis, UCB, Galderma, Bristol Myers Squibb, Boehringer Ingelheim; participation in boards: AbbVie, Almirall, Novartis, Janssen, UCB, Pfizer, Galderma, Biogen, Lilly, Sanofi, Bristol Myers Squibb, Boehringer Ingelheim; GRAPPA Executive Board (elected), British Society for Medical Dermatology (BSMD) Committee. GS: honoraria: Novartis, Janssen. SJWS-W: grant support: Medical Research Council (MR/W027151/1). YT: research grants from Mitsubishi Tanabe, Eisai, Chugai, Taisho; speaking fees and/or honoraria from Eli Lilly, AstraZeneca, AbbVie, Gilead, Chugai, Boehringer Ingelheim, GlaxoSmithKline, Eisai, Taisho, Bristol Myers, Pfizer, Taiho. FEVdB: consultancy honoraria from AbbVie, Amgen, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB. AZ: speakers bureau: AbbVie, Novartis, Janssen, Lilly, UCB, Amgen; paid instructor for AbbVie, Novartis, UCB. DvdH: consulting fees AbbVie, Argenx, Bayer, BMS, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Takeda, UCB Pharma; Director of Imaging Rheumatology bv; Associate Editor for Annals of the Rheumatic Diseases; Editorial Board Member for Journal of Rheumatology and RMD Open; Advisor Assessment Axial Spondyloarthritis International Society. JSS: research grants from AbbVie, AstraZeneca, Lilly, Galapagos; royalties from Elsevier (textbook); consulting fees from AbbVie, Galapagos/Gilead, Novartis-Sandoz, BMS, Samsung, Sanofi, Chugai, R-Pharma, Lilly; honoraria from Samsung, Lilly, R-Pharma, Chugai, MSD, Janssen, Novartis-Sandoz; participation in advisory board from AstraZeneca.
Figures
References
-
- Kerschbaumer A, Smolen JSS, Ferreira JO, et al. . Efficacy and safety of pharmacological treatment of psoriatic arthritis: a systematic literature research informing the 2023 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2024:1–15. doi:ard-2024-225534 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

