Despite the odds: formation of the SARS-CoV-2 methylation complex
- PMID: 38499483
- PMCID: PMC11194070
- DOI: 10.1093/nar/gkae165
Despite the odds: formation of the SARS-CoV-2 methylation complex
Abstract
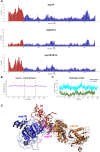
Coronaviruses modify their single-stranded RNA genome with a methylated cap during replication to mimic the eukaryotic mRNAs. The capping process is initiated by several nonstructural proteins (nsp) encoded in the viral genome. The methylation is performed by two methyltransferases, nsp14 and nsp16, while nsp10 acts as a co-factor to both. Additionally, nsp14 carries an exonuclease domain which operates in the proofreading system during RNA replication of the viral genome. Both nsp14 and nsp16 were reported to independently bind nsp10, but the available structural information suggests that the concomitant interaction between these three proteins would be impossible due to steric clashes. Here, we show that nsp14, nsp10, and nsp16 can form a heterotrimer complex upon significant allosteric change. This interaction is expected to encourage the formation of mature capped viral mRNA, modulating nsp14's exonuclease activity, and protecting the viral RNA. Our findings show that nsp14 is amenable to allosteric regulation and may serve as a novel target for therapeutic approaches.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Coronavirus 2'-O-methyltransferase: A promising therapeutic target.Virus Res. 2023 Oct 15;336:199211. doi: 10.1016/j.virusres.2023.199211. Epub 2023 Sep 4. Virus Res. 2023. PMID: 37634741 Free PMC article. Review.
-
Iron-sulfur clusters in SARS-CoV-2 exoribonuclease and methyltransferase complexes: relevance for viral genome proofreading and capping.Nat Commun. 2025 Aug 15;16(1):7585. doi: 10.1038/s41467-025-62832-5. Nat Commun. 2025. PMID: 40817372 Free PMC article.
-
Mn2+ coordinates Cap-0-RNA to align substrates for efficient 2'-O-methyl transfer by SARS-CoV-2 nsp16.Sci Signal. 2021 Jun 29;14(689):eabh2071. doi: 10.1126/scisignal.abh2071. Sci Signal. 2021. PMID: 34131072 Free PMC article.
-
Biochemical and structural insights into the mechanisms of SARS coronavirus RNA ribose 2'-O-methylation by nsp16/nsp10 protein complex.PLoS Pathog. 2011 Oct;7(10):e1002294. doi: 10.1371/journal.ppat.1002294. Epub 2011 Oct 13. PLoS Pathog. 2011. PMID: 22022266 Free PMC article.
-
A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping.Cells. 2020 May 20;9(5):1267. doi: 10.3390/cells9051267. Cells. 2020. PMID: 32443810 Free PMC article. Review.
Cited by
-
A post-assembly conformational change makes the SARS-CoV-2 polymerase elongation-competent.Nucleic Acids Res. 2025 May 22;53(10):gkaf450. doi: 10.1093/nar/gkaf450. Nucleic Acids Res. 2025. PMID: 40464687 Free PMC article.
-
Coronavirus 2'-O-methyltransferase: A promising therapeutic target.Virus Res. 2023 Oct 15;336:199211. doi: 10.1016/j.virusres.2023.199211. Epub 2023 Sep 4. Virus Res. 2023. PMID: 37634741 Free PMC article. Review.
-
A post-assembly conformational change makes the SARS-CoV-2 polymerase elongation-competent.bioRxiv [Preprint]. 2025 Jan 10:2025.01.10.632299. doi: 10.1101/2025.01.10.632299. bioRxiv. 2025. Update in: Nucleic Acids Res. 2025 May 22;53(10):gkaf450. doi: 10.1093/nar/gkaf450. PMID: 39829827 Free PMC article. Updated. Preprint.
-
Refolding of lid subdomain of SARS-CoV-2 nsp14 upon nsp10 interaction releases exonuclease activity.Structure. 2022 Aug 4;30(8):1050-1054.e2. doi: 10.1016/j.str.2022.04.014. Epub 2022 May 23. Structure. 2022. PMID: 35609600 Free PMC article.
-
Oligomeric State of β-Coronavirus Non-Structural Protein 10 Stimulators Studied by Small Angle X-ray Scattering.Int J Mol Sci. 2023 Sep 4;24(17):13649. doi: 10.3390/ijms241713649. Int J Mol Sci. 2023. PMID: 37686452 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

