Molecular insights into the fine-tuning of pH-dependent ArsR-mediated regulation of the SabA adhesin in Helicobacter pylori
- PMID: 38499492
- PMCID: PMC11162790
- DOI: 10.1093/nar/gkae188
Molecular insights into the fine-tuning of pH-dependent ArsR-mediated regulation of the SabA adhesin in Helicobacter pylori
Abstract
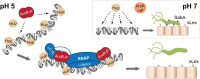
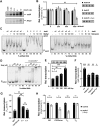
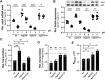
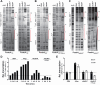
Adaptation to variations in pH is crucial for the ability of Helicobacter pylori to persist in the human stomach. The acid responsive two-component system ArsRS, constitutes the global regulon that responds to acidic conditions, but molecular details of how transcription is affected by the ArsR response regulator remains poorly understood. Using a combination of DNA-binding studies, in vitro transcription assays, and H. pylori mutants, we demonstrate that phosphorylated ArsR (ArsR-P) forms an active protein complex that binds DNA with high specificity in order to affect transcription. Our data showed that DNA topology is key for DNA binding. We found that AT-rich DNA sequences direct ArsR-P to specific sites and that DNA-bending proteins are important for the effect of ArsR-P on transcription regulation. The repression of sabA transcription is mediated by ArsR-P with the support of Hup and is affected by simple sequence repeats located upstream of the sabA promoter. Here stochastic events clearly contribute to the fine-tuning of pH-dependent gene regulation. Our results reveal important molecular aspects for how ArsR-P acts to repress transcription in response to acidic conditions. Such transcriptional control likely mediates shifts in bacterial positioning in the gastric mucus layer.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Repetitive sequence variations in the promoter region of the adhesin-encoding gene sabA of Helicobacter pylori affect transcription.J Bacteriol. 2014 Oct;196(19):3421-9. doi: 10.1128/JB.01956-14. Epub 2014 Jul 14. J Bacteriol. 2014. PMID: 25022855 Free PMC article.
-
Determinants of the regulation of Helicobacter pylori adhesins include repeat sequences in both promoter and coding regions as well as the two-component system ArsRS.J Med Microbiol. 2017 Jun;66(6):798-807. doi: 10.1099/jmm.0.000491. Epub 2017 Jun 9. J Med Microbiol. 2017. PMID: 28598306
-
Delineation of the pH-Responsive Regulon Controlled by the Helicobacter pylori ArsRS Two-Component System.Infect Immun. 2021 Mar 17;89(4):e00597-20. doi: 10.1128/IAI.00597-20. Print 2021 Mar 17. Infect Immun. 2021. PMID: 33526561 Free PMC article.
-
Acid-responsive gene regulation in the human pathogen Helicobacter pylori.J Biotechnol. 2006 Oct 20;126(1):52-60. doi: 10.1016/j.jbiotec.2006.03.045. Epub 2006 May 19. J Biotechnol. 2006. PMID: 16713649 Review.
-
Helicobacter pylori BabA-SabA Key Roles in the Adherence Phase: The Synergic Mechanism for Successful Colonization and Disease Development.Toxins (Basel). 2021 Jul 13;13(7):485. doi: 10.3390/toxins13070485. Toxins (Basel). 2021. PMID: 34357957 Free PMC article. Review.
Cited by
-
Advances in adhesion-related pathogenesis in Mycoplasma pneumoniae infection.Front Microbiol. 2025 Jul 23;16:1613760. doi: 10.3389/fmicb.2025.1613760. eCollection 2025. Front Microbiol. 2025. PMID: 40771685 Free PMC article. Review.
-
Pivotal role of Helicobacter pylori virulence genes in pathogenicity and vaccine development.Front Med (Lausanne). 2025 Jan 6;11:1523991. doi: 10.3389/fmed.2024.1523991. eCollection 2024. Front Med (Lausanne). 2025. PMID: 39850097 Free PMC article. Review.
References
-
- Keilberg D., Ottemann K.M. How Helicobacter pylori senses, targets and interacts with the gastric epithelium. Environ. Microbiol. 2016; 18:791–806. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

