Hepatic nutrient and hormone signaling to mTORC1 instructs the postnatal metabolic zonation of the liver
- PMID: 38499523
- PMCID: PMC10948770
- DOI: 10.1038/s41467-024-46032-1
Hepatic nutrient and hormone signaling to mTORC1 instructs the postnatal metabolic zonation of the liver
Abstract
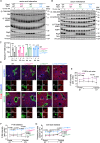
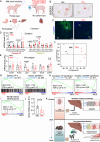
The metabolic functions of the liver are spatially organized in a phenomenon called zonation, linked to the differential exposure of portal and central hepatocytes to nutrient-rich blood. The mTORC1 signaling pathway controls cellular metabolism in response to nutrients and insulin fluctuations. Here we show that simultaneous genetic activation of nutrient and hormone signaling to mTORC1 in hepatocytes results in impaired establishment of postnatal metabolic and zonal identity of hepatocytes. Mutant hepatocytes fail to upregulate postnatally the expression of Frizzled receptors 1 and 8, and show reduced Wnt/β-catenin activation. This defect, alongside diminished paracrine Wnt2 ligand expression by endothelial cells, underlies impaired postnatal maturation. Impaired zonation is recapitulated in a model of constant supply of nutrients by parenteral nutrition to piglets. Our work shows the role of hepatocyte sensing of fluctuations in nutrients and hormones for triggering a latent metabolic zonation program.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice.Hepatology. 2018 Aug;68(2):707-722. doi: 10.1002/hep.29613. Epub 2018 Feb 1. Hepatology. 2018. PMID: 29059455 Free PMC article.
-
Transcription dynamics in a physiological process: β-catenin signaling directs liver metabolic zonation.Int J Biochem Cell Biol. 2011 Feb;43(2):271-8. doi: 10.1016/j.biocel.2009.11.004. Epub 2009 Nov 13. Int J Biochem Cell Biol. 2011. PMID: 19914393 Review.
-
Hepatic mTORC1 signaling activates ATF4 as part of its metabolic response to feeding and insulin.Mol Metab. 2021 Nov;53:101309. doi: 10.1016/j.molmet.2021.101309. Epub 2021 Jul 23. Mol Metab. 2021. PMID: 34303878 Free PMC article.
-
Pre-clinical and clinical investigations of metabolic zonation in liver diseases: The potential of microphysiology systems.Exp Biol Med (Maywood). 2017 Oct;242(16):1605-1616. doi: 10.1177/1535370217707731. Epub 2017 May 3. Exp Biol Med (Maywood). 2017. PMID: 28467181 Free PMC article. Review.
-
Inhibiting Glutamine-Dependent mTORC1 Activation Ameliorates Liver Cancers Driven by β-Catenin Mutations.Cell Metab. 2019 May 7;29(5):1135-1150.e6. doi: 10.1016/j.cmet.2019.01.002. Epub 2019 Jan 31. Cell Metab. 2019. PMID: 30713111 Free PMC article.
Cited by
-
Defined Diets Link Iron and α-Linolenic Acid to Cyp1b1 Regulation of Neonatal Liver Development Through Srebp Forms and LncRNA H19.Int J Mol Sci. 2025 Feb 25;26(5):2011. doi: 10.3390/ijms26052011. Int J Mol Sci. 2025. PMID: 40076634 Free PMC article.
-
A mild increase in nutrient signaling to mTORC1 in mice leads to parenchymal damage, myeloid inflammation and shortened lifespan.Nat Aging. 2024 Aug;4(8):1102-1120. doi: 10.1038/s43587-024-00635-x. Epub 2024 Jun 7. Nat Aging. 2024. PMID: 38849535 Free PMC article.
-
Hepatic stellate cells control liver zonation, size and functions via R-spondin 3.Nature. 2025 Apr;640(8059):752-761. doi: 10.1038/s41586-025-08677-w. Epub 2025 Mar 12. Nature. 2025. PMID: 40074890 Free PMC article.
-
HSDL2 links nutritional cues to bile acid and cholesterol homeostasis.Sci Adv. 2024 May 31;10(22):eadk9681. doi: 10.1126/sciadv.adk9681. Epub 2024 May 31. Sci Adv. 2024. PMID: 38820148 Free PMC article.
References
-
- Martini, T., Naef, F. & Tchorz, J. S. Spatiotemporal Metabolic Liver Zonation and Consequences on Pathophysiology. Ann. Rev. Pathol. Mechan. Dis.1810.1146/annurev-pathmechdis-031521-024831 (2023). - PubMed
MeSH terms
Substances
Grants and funding
- ERC-2014-STG-638891/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- PID2022-136413OB-I00/Ministry of Economy and Competitiveness | Agencia Estatal de Investigación (Spanish Agencia Estatal de Investigación)
- Young Investigator Programme/European Molecular Biology Organization (EMBO)
- LCF/PR/HR21/0046)./"la Caixa" Foundation (Caixa Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

