Progression free survival of myeloma patients who become IFE-negative correlates with the detection of residual monoclonal free light chain (FLC) by mass spectrometry
- PMID: 38499538
- PMCID: PMC10948753
- DOI: 10.1038/s41408-024-00995-y
Progression free survival of myeloma patients who become IFE-negative correlates with the detection of residual monoclonal free light chain (FLC) by mass spectrometry
Abstract
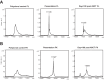
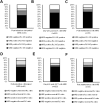
Deeper responses are associated with improved survival in patients being treated for myeloma. However, the sensitivity of the current blood-based assays is limited. Historical studies suggested that normalisation of the serum free light chain (FLC) ratio in patients who were negative by immunofixation electrophoresis (IFE) was associated with improved outcomes. However, recently this has been called into question. Mass spectrometry (MS)-based FLC assessments may offer a superior methodology for the detection of monoclonal FLC due to greater sensitivity. To test this hypothesis, all available samples from patients who were IFE negative after treatment with carfilzomib and lenalidomide-based induction and autologous stem cell transplantation (ASCT) in the Myeloma XI trial underwent FLC-MS testing. FLC-MS response assessments from post-induction, day+100 post-ASCT and six months post-maintenance randomisation were compared to serum FLC assay results. Almost 40% of patients had discordant results and 28.7% of patients with a normal FLC ratio had residual monoclonal FLC detectable by FLC-MS. FLC-MS positivity was associated with reduced progression-free survival (PFS) but an abnormal FLC ratio was not. This study demonstrates that FLC-MS provides a superior methodology for the detection of residual monoclonal FLC with FLC-MS positivity identifying IFE-negative patients who are at higher risk of early progression.
© 2024. The Author(s).
Conflict of interest statement
HVG has received research funding from The Binding Site Ltd, part of Thermo Fisher Scientific. GP is on the medical advisory board and has received educational funding from Janssen Oncology; BMS-Celgene; Amgen; Takeda; The Binding Site Ltd part of Thermo Fisher Scientific; Sanofi/Aventis; Beigene; and GlaxoSmithKline.
Figures




Similar articles
-
Mass spectrometry vs immunofixation for treatment monitoring in multiple myeloma.Blood Adv. 2022 Jun 14;6(11):3234-3239. doi: 10.1182/bloodadvances.2021006762. Blood Adv. 2022. PMID: 35157768 Free PMC article. Clinical Trial.
-
Assessing the efficiency of free light chain assay in monitoring patients with multiple myeloma before and after autologous stem cell transplantation along with serum protein electrophoresis and serum protein immunofixation.Roum Arch Microbiol Immunol. 2011 Jan-Mar;70(1):15-22. Roum Arch Microbiol Immunol. 2011. PMID: 21717807
-
Prognostic Implications of Rising Serum Monoclonal Protein and Free Light Chains after Autologous Stem Cell Transplantation in Patients with Multiple Myeloma.Transplant Cell Ther. 2021 Apr;27(4):309.e1-309.e5. doi: 10.1016/j.jtct.2020.11.022. Epub 2020 Dec 17. Transplant Cell Ther. 2021. PMID: 33836869
-
The evolving use of serum free light chain assays in haematology.Br J Haematol. 2008 May;141(4):413-22. doi: 10.1111/j.1365-2141.2008.07079.x. Epub 2008 Mar 3. Br J Haematol. 2008. PMID: 18318757 Review.
-
International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders.Leukemia. 2009 Feb;23(2):215-24. doi: 10.1038/leu.2008.307. Epub 2008 Nov 20. Leukemia. 2009. PMID: 19020545 Review.
Cited by
-
[A protein-specific quantitative detection method based on polyacrylamide gel electrophoresis and online fluorescence imaging].Se Pu. 2025 Sep;43(9):1070-1077. doi: 10.3724/SP.J.1123.2024.12017. Se Pu. 2025. PMID: 40910314 Free PMC article. Chinese.
-
Minimal Residual Disease Negativity as the Primary Goal of Multiple Myeloma Therapy.Drugs. 2025 Sep 4. doi: 10.1007/s40265-025-02232-7. Online ahead of print. Drugs. 2025. PMID: 40908381 Review.
-
Monoclonal immunoglobulin measurement by mass spectrometry in patients with multiple myeloma and kidney failure: Analysis from the EuLITE trial.Br J Haematol. 2025 May 25;207(1):294-8. doi: 10.1111/bjh.20168. Online ahead of print. Br J Haematol. 2025. PMID: 40415308 Free PMC article. No abstract available.
-
Screening for monoclonal gammopathy of undetermined significance is contraindicated.Br J Haematol. 2025 Jul;207(1):43-45. doi: 10.1111/bjh.20175. Epub 2025 May 22. Br J Haematol. 2025. PMID: 40400390 Free PMC article. Review.
-
EHA-EMN Evidence-Based Guidelines for diagnosis, treatment and follow-up of patients with multiple myeloma.Nat Rev Clin Oncol. 2025 Sep;22(9):680-700. doi: 10.1038/s41571-025-01041-x. Epub 2025 Jul 7. Nat Rev Clin Oncol. 2025. PMID: 40624367 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

