Nuclei-specific hypothalamus networks predict a dimensional marker of stress in humans
- PMID: 38499548
- PMCID: PMC10948785
- DOI: 10.1038/s41467-024-46275-y
Nuclei-specific hypothalamus networks predict a dimensional marker of stress in humans
Abstract
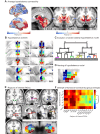
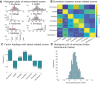
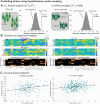
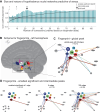
The hypothalamus is part of the hypothalamic-pituitary-adrenal axis which activates stress responses through release of cortisol. It is a small but heterogeneous structure comprising multiple nuclei. In vivo human neuroimaging has rarely succeeded in recording signals from individual hypothalamus nuclei. Here we use human resting-state fMRI (n = 498) with high spatial resolution to examine relationships between the functional connectivity of specific hypothalamic nuclei and a dimensional marker of prolonged stress. First, we demonstrate that we can parcellate the human hypothalamus into seven nuclei in vivo. Using the functional connectivity between these nuclei and other subcortical structures including the amygdala, we significantly predict stress scores out-of-sample. Predictions use 0.0015% of all possible brain edges, are specific to stress, and improve when using nucleus-specific compared to whole-hypothalamus connectivity. Thus, stress relates to connectivity changes in precise and functionally meaningful subcortical networks, which may be exploited in future studies using interventions in stress disorders.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation.J Abnorm Psychol. 2015 Nov;124(4):817-33. doi: 10.1037/abn0000094. J Abnorm Psychol. 2015. PMID: 26595470 Free PMC article.
-
Resting amygdala connectivity and basal sympathetic tone as markers of chronic hypervigilance.Psychoneuroendocrinology. 2019 Apr;102:68-78. doi: 10.1016/j.psyneuen.2018.11.036. Epub 2018 Nov 26. Psychoneuroendocrinology. 2019. PMID: 30529716 Free PMC article.
-
Identification of biopsychological trait markers in functional neurological disorders.Brain. 2023 Jun 1;146(6):2627-2641. doi: 10.1093/brain/awac442. Brain. 2023. PMID: 36417451 Free PMC article.
-
The brain and the stress axis: the neural correlates of cortisol regulation in response to stress.Neuroimage. 2009 Sep;47(3):864-71. doi: 10.1016/j.neuroimage.2009.05.074. Epub 2009 Jun 13. Neuroimage. 2009. PMID: 19500680 Review.
-
Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes.J Neuroendocrinol. 2002 Jun;14(6):506-13. doi: 10.1046/j.1365-2826.2002.00798.x. J Neuroendocrinol. 2002. PMID: 12047726 Review.
Cited by
-
Editorial: Noninvasive brain stimulation: a promising approach to study and improve emotion regulation.Front Behav Neurosci. 2025 Jun 23;19:1633936. doi: 10.3389/fnbeh.2025.1633936. eCollection 2025. Front Behav Neurosci. 2025. PMID: 40630047 Free PMC article. No abstract available.
-
MRI-Based Structural Development of the Human Newborn Hypothalamus.bioRxiv [Preprint]. 2025 Jun 21:2025.06.20.660741. doi: 10.1101/2025.06.20.660741. bioRxiv. 2025. PMID: 40667207 Free PMC article. Preprint.
-
The effect of live-performed music therapy with physical contact in preterm infants on parental perceived stress and salivary cortisol levels.Front Psychol. 2024 Oct 7;15:1441824. doi: 10.3389/fpsyg.2024.1441824. eCollection 2024. Front Psychol. 2024. PMID: 39434912 Free PMC article.
-
The insular cortex, autonomic asymmetry and cardiovascular control: looking at the right side of stroke.Clin Auton Res. 2024 Dec;34(6):549-560. doi: 10.1007/s10286-024-01066-9. Epub 2024 Sep 24. Clin Auton Res. 2024. PMID: 39316247 Review.
-
Cortical and subcortical mapping of the allostatic-interoceptive system in the human brain using 7 Tesla fMRI.bioRxiv [Preprint]. 2025 Jan 13:2023.07.20.548178. doi: 10.1101/2023.07.20.548178. bioRxiv. 2025. PMID: 37546889 Free PMC article. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

