Multichannel bridges and NSC synergize to enhance axon regeneration, myelination, synaptic reconnection, and recovery after SCI
- PMID: 38499577
- PMCID: PMC10948859
- DOI: 10.1038/s41536-024-00356-0
Multichannel bridges and NSC synergize to enhance axon regeneration, myelination, synaptic reconnection, and recovery after SCI
Abstract
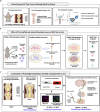
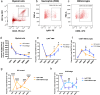
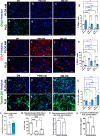
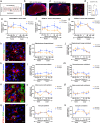
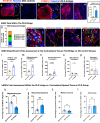
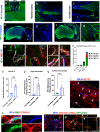
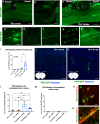
Regeneration in the injured spinal cord is limited by physical and chemical barriers. Acute implantation of a multichannel poly(lactide-co-glycolide) (PLG) bridge mechanically stabilizes the injury, modulates inflammation, and provides a permissive environment for rapid cellularization and robust axonal regrowth through this otherwise inhibitory milieu. However, without additional intervention, regenerated axons remain largely unmyelinated (<10%), limiting functional repair. While transplanted human neural stem cells (hNSC) myelinate axons after spinal cord injury (SCI), hNSC fate is highly influenced by the SCI inflammatory microenvironment, also limiting functional repair. Accordingly, we investigated the combination of PLG scaffold bridges with hNSC to improve histological and functional outcome after SCI. In vitro, hNSC culture on a PLG scaffold increased oligodendroglial lineage selection after inflammatory challenge. In vivo, acute PLG bridge implantation followed by chronic hNSC transplantation demonstrated a robust capacity of donor human cells to migrate into PLG bridge channels along regenerating axons and integrate into the host spinal cord as myelinating oligodendrocytes and synaptically integrated neurons. Axons that regenerated through the PLG bridge formed synaptic circuits that connected the ipsilateral forelimb muscle to contralateral motor cortex. hNSC transplantation significantly enhanced the total number of regenerating and myelinated axons identified within the PLG bridge. Finally, the combination of acute bridge implantation and hNSC transplantation exhibited robust improvement in locomotor recovery. These data identify a successful strategy to enhance neurorepair through a temporally layered approach using acute bridge implantation and chronic cell transplantation to spare tissue, promote regeneration, and maximize the function of new axonal connections.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Update of
-
Multichannel bridges and NSC synergize to enhance axon regeneration, myelination, synaptic reconnection, and recovery after SCI.Res Sq [Preprint]. 2023 Jul 19:rs.3.rs-3044426. doi: 10.21203/rs.3.rs-3044426/v1. Res Sq. 2023. Update in: NPJ Regen Med. 2024 Mar 18;9(1):12. doi: 10.1038/s41536-024-00356-0. PMID: 37502943 Free PMC article. Updated. Preprint.
Similar articles
-
Multichannel bridges and NSC synergize to enhance axon regeneration, myelination, synaptic reconnection, and recovery after SCI.Res Sq [Preprint]. 2023 Jul 19:rs.3.rs-3044426. doi: 10.21203/rs.3.rs-3044426/v1. Res Sq. 2023. Update in: NPJ Regen Med. 2024 Mar 18;9(1):12. doi: 10.1038/s41536-024-00356-0. PMID: 37502943 Free PMC article. Updated. Preprint.
-
Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: Association with recovery of forelimb function.Biomaterials. 2015 Oct;65:1-12. doi: 10.1016/j.biomaterials.2015.05.032. Epub 2015 Jun 23. Biomaterials. 2015. PMID: 26134079 Free PMC article.
-
PLG Bridge Implantation in Chronic SCI Promotes Axonal Elongation and Myelination.ACS Biomater Sci Eng. 2019 Dec 9;5(12):6679-6690. doi: 10.1021/acsbiomaterials.9b01012. Epub 2019 Nov 14. ACS Biomater Sci Eng. 2019. PMID: 33423486 Free PMC article.
-
Schwann cell transplantation for spinal cord injury repair: its significant therapeutic potential and prospectus.Rev Neurosci. 2015;26(2):121-8. doi: 10.1515/revneuro-2014-0068. Rev Neurosci. 2015. PMID: 25581750 Review.
-
Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury.Handb Clin Neurol. 2012;109:523-40. doi: 10.1016/B978-0-444-52137-8.00032-2. Handb Clin Neurol. 2012. PMID: 23098734 Review.
Cited by
-
Exosomes: a promising microenvironment modulator for spinal cord injury treatment.Int J Biol Sci. 2025 Jun 5;21(8):3791-3824. doi: 10.7150/ijbs.115242. eCollection 2025. Int J Biol Sci. 2025. PMID: 40520019 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

