Development strategy of non-GMO organism for increased hemoproteins in Corynebacterium glutamicum: a growth-acceleration-targeted evolution
- PMID: 38499686
- PMCID: PMC11003892
- DOI: 10.1007/s00449-024-02986-6
Development strategy of non-GMO organism for increased hemoproteins in Corynebacterium glutamicum: a growth-acceleration-targeted evolution
Abstract
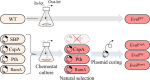
Heme, found in hemoproteins, is a valuable source of iron, an essential mineral. The need for an alternative hemoprotein source has emerged due to the inherent risks of large-scale livestock farming and animal proteins. Corynebacterium glutamicum, regarded for Qualified Presumption of Safety or Generally Recognized as Safe, can biosynthesize hemoproteins. C. glutamicum single-cell protein (SCP) can be a valuable alternative hemoprotein for supplying heme iron without adversely affecting blood fat levels. We constructed the chemostat culture system to increase hemoprotein content in C. glutamicum SCP. Through adaptive evolution, hemoprotein levels could be naturally increased to address oxidative stress resulting from enhanced growth rate. In addition, we used several specific plasmids containing growth-accelerating genes and the hemA promoter to expedite the evolutionary process. Following chemostat culture for 15 days, the plasmid in selected descendants was cured. The evolved strains showed improved specific growth rates from 0.59 h-1 to 0.62 h-1, 20% enhanced resistance to oxidative stress, and increased heme concentration from 12.95 µg/g-DCW to 14.22-15.24 µg/g-DCW. Notably, the putative peptidyl-tRNA hydrolase-based evolved strain manifested the most significant increase (30%) of hemoproteins. This is the first report presenting the potential of a growth-acceleration-targeted evolution (GATE) strategy for developing non-GMO industrial strains with increased bio-product productivity.
Keywords: Corynebacterium glutamicum; Adaptive evolution; Hemoproteins; Positive feedback.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study and the interpretation of data.
Figures




References
-
- Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671–678. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

