High-throughput characterization of bacterial responses to complex mixtures of chemical pollutants
- PMID: 38499812
- PMCID: PMC10994839
- DOI: 10.1038/s41564-024-01626-9
High-throughput characterization of bacterial responses to complex mixtures of chemical pollutants
Abstract
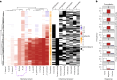
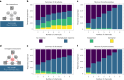
Our understanding of how microbes respond to micropollutants, such as pesticides, is almost wholly based on single-species responses to individual chemicals. However, in natural environments, microbes experience multiple pollutants simultaneously. Here we perform a matrix of multi-stressor experiments by assaying the growth of model and non-model strains of bacteria in all 255 combinations of 8 chemical stressors (antibiotics, herbicides, fungicides and pesticides). We found that bacterial strains responded in different ways to stressor mixtures, which could not be predicted simply from their phylogenetic relatedness. Increasingly complex chemical mixtures were both more likely to negatively impact bacterial growth in monoculture and more likely to reveal net interactive effects. A mixed co-culture of strains proved more resilient to increasingly complex mixtures and revealed fewer interactions in the growth response. These results show predictability in microbial population responses to chemical stressors and could increase the utility of next-generation eco-toxicological assays.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil.Environ Toxicol Pharmacol. 2017 Oct;55:20-29. doi: 10.1016/j.etap.2017.08.001. Epub 2017 Aug 7. Environ Toxicol Pharmacol. 2017. PMID: 28806580 Review.
-
Toxicity on the luminescent bacterium Vibrio fischeri (Beijerinck). II: Response to complex mixtures of heterogeneous chemicals at low levels of individual components.Ecotoxicol Environ Saf. 2012 Dec;86:93-100. doi: 10.1016/j.ecoenv.2012.08.030. Epub 2012 Oct 22. Ecotoxicol Environ Saf. 2012. PMID: 23084651
-
Interactive effects of an insecticide and a fungicide on different organism groups and ecosystem functioning in a stream detrital food web.Aquat Toxicol. 2017 May;186:215-221. doi: 10.1016/j.aquatox.2017.03.008. Epub 2017 Mar 9. Aquat Toxicol. 2017. PMID: 28324829
-
Chiral pesticides: identification, description, and environmental implications.Rev Environ Contam Toxicol. 2012;217:1-74. doi: 10.1007/978-1-4614-2329-4_1. Rev Environ Contam Toxicol. 2012. PMID: 22350557 Review.
-
Toxicological interactions of pesticide mixtures: an update.Arch Toxicol. 2017 Oct;91(10):3211-3223. doi: 10.1007/s00204-017-2043-5. Epub 2017 Aug 28. Arch Toxicol. 2017. PMID: 28845507 Review.
Cited by
-
Number and dissimilarity of global change factors influences soil properties and functions.Nat Commun. 2024 Sep 18;15(1):8188. doi: 10.1038/s41467-024-52511-2. Nat Commun. 2024. PMID: 39294171 Free PMC article.
-
Phylogenetic clustering of microbial communities as a biomarker for chemical pollution.FEMS Microbiol Ecol. 2025 Apr 14;101(5):fiaf047. doi: 10.1093/femsec/fiaf047. FEMS Microbiol Ecol. 2025. PMID: 40317081 Free PMC article.
-
Translating microbial kinetics into quantitative responses and testable hypotheses using Kinbiont.Nat Commun. 2025 Jul 11;16(1):6440. doi: 10.1038/s41467-025-61592-6. Nat Commun. 2025. PMID: 40645927 Free PMC article.
References
-
- Thompson MSA, et al. Gene-to-ecosystem impacts of a catastrophic pesticide spill: testing a multilevel bioassessment approach in a river ecosystem. Freshw. Biol. 2016;61:2037–2050. doi: 10.1111/fwb.12676. - DOI
-
- Gautam, K. & Anbumani, S. Ecotoxicological effects of organic micro-pollutants on the environment. in Current Developments in Biotechnology and Bioengineering 481–501 (Elsevier, 2020); 10.1016/b978-0-12-819594-9.00019-x
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

