Literature Survey for In-Vivo Reynolds and Womersley Numbers of Various Arteries and Implications for Compliant In-Vitro Modelling
- PMID: 38499933
- PMCID: PMC11319390
- DOI: 10.1007/s13239-024-00723-4
Literature Survey for In-Vivo Reynolds and Womersley Numbers of Various Arteries and Implications for Compliant In-Vitro Modelling
Abstract
Purpose: In-vitro modelling can be used to investigate haemodynamics of arterial geometry and stent implants. However, in-vitro model fidelity relies on precise matching of in-vivo conditions. In pulsatile flow, velocity distribution and wall shear stress depend on compliance, and the Reynolds and Womersley numbers. However, matching such values may lead to unachievable tolerances in phantom fabrication.
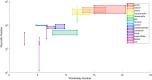
Methods: Published Reynolds and Womersley numbers for 14 major arteries in the human body were determined via a literature search. Preference was given to in-vivo publications but in-vitro and in-silico values were presented when in-vivo values were not found. Subsequently ascending aorta and carotid artery case studies were presented to highlight the limitations dynamic matching would apply to phantom fabrication.
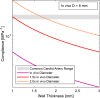
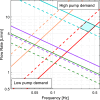
Results: Seven studies reported the in-vivo Reynolds and Womersley numbers for the aorta and two for the carotid artery. However, only one study each reported in-vivo numbers for the remaining ten arteries. No in-vivo data could be found for the femoral, superior mesenteric and renal arteries. Thus, information derived in-vitro and in-silico were provided instead. The ascending aorta and carotid artery models required scaling to 1.5× and 3× life-scale, respectively, to achieve dimensional tolerance restrictions. Modelling the ascending aorta with the comparatively high viscosity water/glycerine solution will lead to high pump power demands. However, all the working fluids considered could be dynamically matched with low pump demand for the carotid model.
Conclusion: This paper compiles available human haemodynamic information, and highlights the paucity of information for some arteries. It also provides a method for optimal in-vitro experimental configuration.
Keywords: Cardiovascular research; Dynamic matching; Haemodynamics; Insitu modelling; Phantom modelling.
© 2024. The Author(s).
Conflict of interest statement
The authors have no financial interest in the outcomes of this study.
Figures



References
-
- World Health Organization (WHO). Cardiovascular Diseases (CVDs): Fact Sheet No. 317. 2012. Geneva: World Health Organization, 2012.
-
- Raffel, M., et al. Particle Image Velocimetry: A Practical Guide, 3rd ed. (No. Book, Whole). Cham: Springer International Publishing, 2018.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

