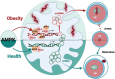
AMPK Suppression Due to Obesity Drives Oocyte mtDNA Heteroplasmy via ATF5-POLG Axis
- PMID: 38499990
- PMCID: PMC11132083
- DOI: 10.1002/advs.202307480
AMPK Suppression Due to Obesity Drives Oocyte mtDNA Heteroplasmy via ATF5-POLG Axis
Abstract
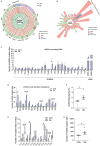
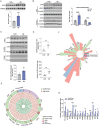
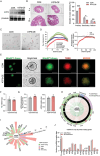
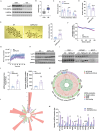
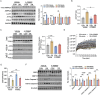
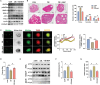
Due to the exclusive maternal transmission, oocyte mitochondrial dysfunction reduces fertility rates, affects embryonic development, and programs offspring to metabolic diseases. However, mitochondrial DNA (mtDNA) are vulnerable to mutations during oocyte maturation, leading to mitochondrial nucleotide variations (mtSNVs) within a single oocyte, referring to mtDNA heteroplasmy. Obesity (OB) accounts for more than 40% of women at the reproductive age in the USA, but little is known about impacts of OB on mtSNVs in mature oocytes. It is found that OB reduces mtDNA content and increases mtSNVs in mature oocytes, which impairs mitochondrial energetic functions and oocyte quality. In mature oocytes, OB suppresses AMPK activity, aligned with an increased binding affinity of the ATF5-POLG protein complex to mutated mtDNA D-loop and protein-coding regions. Similarly, AMPK knockout increases the binding affinity of ATF5-POLG proteins to mutated mtDNA, leading to the replication of heteroplasmic mtDNA and impairing oocyte quality. Consistently, AMPK activation blocks the detrimental impacts of OB by preventing ATF5-POLG protein recruitment, improving oocyte maturation and mitochondrial energetics. Overall, the data uncover key features of AMPK activation in suppressing mtSNVs, and improving mitochondrial biogenesis and oocyte maturation in obese females.
Keywords: AMPK; female obesity; mature oocyte; mtDNA heteroplasmy.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
