European expert consensus recommendations on the primary care use of direct oral anticoagulants in patients with venous thromboembolism
- PMID: 38500048
- PMCID: PMC10946109
- DOI: 10.1186/s12875-024-02314-7
European expert consensus recommendations on the primary care use of direct oral anticoagulants in patients with venous thromboembolism
Abstract
Background: Direct oral anticoagulants for the treatment of venous thromboembolism are supported by robust clinical trial evidence. Despite published guidance, general practitioners are faced with increasingly complex decisions and implementation remains sub-optimal in certain real-world scenarios.
Methods: A two stage formal consensus exercise was performed to formulate consensus statements and a summary guide, facilitating optimal management of direct oral anticoagulants in venous thromboembolism patients by generalist physicians across Europe. An online questionnaire distributed to a broad panel (Phase 1), followed by a virtual panel discussion by an expert group (Phase 2) were conducted. Phase 1 statements covered nine management domains, and were developed via a literature review and expert steering committee. Participants rated statements by their level of agreement. Phase 1 responses were collated and analysed prior to discussion and iterative refinement in Phase 2.
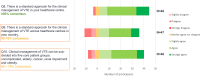
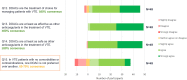
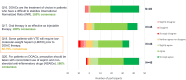
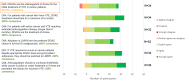
Results: In total 56 participants from across Europe responded to Phase 1. The majority had experience working as general practitioners. Consensus indicated that direct oral anticoagulants are the treatment of choice for managing patients with venous thromboembolism, at initiation and for extended treatment, with a review at three to six months to re-assess treatment effect and risk profile. Direct oral anticoagulant choice should be based on individual patient factors and include shared treatment choice between clinicians and patients; the only sub-group of patients requiring specific guidance are those with cancer.
Conclusion: Results demonstrate an appreciation of best practices, but highlight challenges in clinical practice. The patient pathway and consensus recommendations provided, aim to highlight key considerations for general practice decision making, and aid optimal venous thromboembolism treatment.
Keywords: Cancer associated thromboembolism, Primary Care; Direct oral anticoagulants (DOACs); Formal consensus; Venous thromboembolism (VTE).
© 2024. The Author(s).
Conflict of interest statement
PC is an employee of HEOR Ltd who were paid consultants to Bristol Myers Squibb and Pfizer in connection with the development of this manuscript AF has received honoraria for lectures given for Bayer, Daiichi-Sanyko, Pfizer/BMS and Boehringer-Ingelheim. SH has received personal fees from Bayer, BMS, Daiichi-Sankyo, Pfizer and Sanofi. ES has received consultancy fees from Amal Therapeutics, Aptitude Health, Astellas, AstraZeneca, Beigene, BMS, Celgene, Elsevier, Everest Clinical Research, First Word Group, Five Prime Therapeutics, Gritstone Oncology, Imedex, Merck, My Personal Therapeutics, Novartis, Roche, Sai-Med, Servier and Zymeworks. ES has also received institutional research support from Astellas, AstraZeneca, Roche, MSD and Basilea. AstraZeneca and BMS. FC has received moderate speaker and consulting fees from Daiichi Sankyo. RB has received personal fees from Bayer, BMS, Daiichi-Sankyo and Pfizer. FDRH has received occasional speaker or consultancy fees from Astra-Zeneca, BI, BMS/Pfizer, Bayer and Novartis. RH has also been awarded institutional research funding from Novartis and AstraZeneca. CB has nothing to declare.
Figures

References
-
- McNeill S, Bagot CN. Prevention and treatment of venous thromboembolic disease. Prescriber 2018;29.
-
- Scottish Intercollegiate Guidelines Network (SIGN). Prevention and management of venous thromboembolism. A national clinical guideline Edinburgh [publication number 122]2010 [updated 2014. Available from: https://www.sign.ac.uk/our-guidelines/prevention-and-management-of-venou....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
