Stem cell secretome treatment improves whole-body metabolism, reduces adiposity, and promotes skeletal muscle function in aged mice
- PMID: 38500398
- PMCID: PMC11296109
- DOI: 10.1111/acel.14144
Stem cell secretome treatment improves whole-body metabolism, reduces adiposity, and promotes skeletal muscle function in aged mice
Abstract
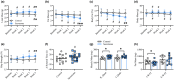
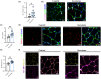
Aging coincides with the progressive loss of muscle mass and strength, increased adiposity, and diminished physical function. Accordingly, interventions aimed at improving muscle, metabolic, and/or physical health are of interest to mitigate the adverse effects of aging. In this study, we tested a stem cell secretome product, which contains extracellular vesicles and growth, cytoskeletal remodeling, and immunomodulatory factors. We examined the effects of 4 weeks of 2×/week unilateral intramuscular secretome injections (quadriceps) in ambulatory aged male C57BL/6 mice (22-24 months) compared to saline-injected aged-matched controls. Secretome delivery substantially increased whole-body lean mass and decreased fat mass, corresponding to higher myofiber cross-sectional area and smaller adipocyte size, respectively. Secretome-treated mice also had greater whole-body physical function (grip strength and rotarod performance) and had higher energy expenditure and physical activity levels compared to control mice. Furthermore, secretome-treated mice had greater skeletal muscle Pax7+ cell abundance, capillary density, collagen IV turnover, reduced intramuscular lipids, and greater Akt and hormone sensitive lipase phosphorylation in adipose tissue. Finally, secretome treatment in vitro directly enhanced muscle cell growth and IL-6 production, and in adipocytes, it reduced lipid content and improved insulin sensitivity. Moreover, indirect treatment with secretome-treated myotube culture media also enhanced muscle cell growth and adipocyte size reduction. Together, these data suggest that intramuscular treatment with a stem cell secretome improves whole-body metabolism, physical function, and remodels skeletal muscle and adipose tissue in aged mice.
Keywords: adipogenesis; fibrosis; lipids; metabolic rate; sarcopenia; stem cells.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
H.S.K., T.E.L., G.N., N.C.B., and S.G. are employed by Immunis. M.J.D. serves on the Immunis scientific advisory board.
Figures




References
-
- Amankwaah, A. F. , Hudson, J. L. , Kim, J. E. , & Campbell, W. W. (2019). Reductions in whole‐body fat mass but not increases in lean mass predict changes in cardiometabolic health indices with exercise training among weight‐stable adults. Nutrition Research, 63, 63–69. 10.1016/j.nutres.2018.11.004 - DOI - PubMed
-
- Bergman, B. C. , Perreault, L. , Strauss, A. , Bacon, S. , Kerege, A. , Harrison, K. , Brozinick, J. T. , Hunerdosse, D. M. , Playdon, M. C. , Holmes, W. , Bui, H. H. , Sanders, P. , Siddall, P. , Wei, T. , Thomas, M. K. , Kuo, M. S. , & Eckel, R. H. (2018). Intramuscular triglyceride synthesis: Importance in muscle lipid partitioning in humans. American Journal of Physiology. Endocrinology and Metabolism, 314(2), E152–E164. 10.1152/ajpendo.00142.2017 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

