Early-Onset Hearing Loss in Mouse Models of Alzheimer's Disease and Increased DNA Damage in the Cochlea
- PMID: 38500536
- PMCID: PMC10948084
- DOI: 10.59368/agingbio.20240025
Early-Onset Hearing Loss in Mouse Models of Alzheimer's Disease and Increased DNA Damage in the Cochlea
Abstract
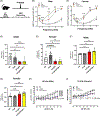
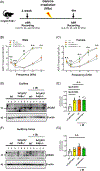
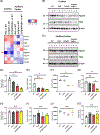
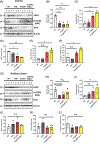
There is considerable interest in whether sensory deficiency is associated with the development of Alzheimer's disease (AD). Notably, the relationship between hearing impairment and AD is of high relevance but still poorly understood. In this study, we found early-onset hearing loss in two AD mouse models, 3xTgAD and 3xTgAD/Polβ+/-. The 3xTgAD/Polβ+/- mouse is DNA repair deficient and has more humanized AD features than the 3xTgAD. Both AD mouse models showed increased auditory brainstem response (ABR) thresholds between 16 and 32 kHz at 4 weeks of age, much earlier than any AD cognitive and behavioral changes. The ABR thresholds were significantly higher in 3xTgAD/Polβ+/- mice than in 3xTgAD mice at 16 kHz, and distortion product otoacoustic emission signals were reduced, indicating that DNA damage may be a factor underlying early hearing impairment in AD. Poly ADP-ribosylation and protein expression levels of DNA damage markers increased significantly in the cochlea of the AD mice but not in the adjacent auditory cortex. Phosphoglycerate mutase 2 levels and the number of synaptic ribbons in the presynaptic zones of inner hair cells were decreased in the cochlea of the AD mice. Furthermore, the activity of sirtuin 3 was downregulated in the cochlea of these mice, indicative of impaired mitochondrial function. Taken together, these findings provide new insights into potential mechanisms for hearing dysfunction in AD and suggest that DNA damage in the cochlea might contribute to the development of early hearing loss in AD.
Conflict of interest statement
Conflict of Interest Statement V.A.B. had a material CRADA agreement with ChromaDex, Inc.
Figures



Similar articles
-
NAD+ supplementation normalizes key Alzheimer's features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency.Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):E1876-E1885. doi: 10.1073/pnas.1718819115. Epub 2018 Feb 5. Proc Natl Acad Sci U S A. 2018. PMID: 29432159 Free PMC article.
-
DNA polymerase β decrement triggers death of olfactory bulb cells and impairs olfaction in a mouse model of Alzheimer's disease.Aging Cell. 2017 Feb;16(1):162-172. doi: 10.1111/acel.12541. Epub 2016 Sep 30. Aging Cell. 2017. PMID: 27686631 Free PMC article.
-
Physiological and Histological Evaluations of the Cochlea between 3xTg-AD Mouse Model of Alzheimer's Diseases and R6/2 Mouse Model of Huntington's Diseases.Chin J Physiol. 2015 Dec 31;58(6):359-66. doi: 10.4077/CJP.2015.BAD334. Chin J Physiol. 2015. PMID: 26717914
-
Ototoxicity: a high risk to auditory function that needs to be monitored in drug development.Front Mol Neurosci. 2024 May 2;17:1379743. doi: 10.3389/fnmol.2024.1379743. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38756707 Free PMC article. Review.
-
Genetic influences on susceptibility of the auditory system to aging and environmental factors.Scand Audiol Suppl. 1992;36:1-39. Scand Audiol Suppl. 1992. PMID: 1488615 Review.
Cited by
-
An Open-Source Deep Learning-Based GUI Toolbox for Automated Auditory Brainstem Response Analyses (ABRA).Res Sq [Preprint]. 2025 Jun 20:rs.3.rs-6735294. doi: 10.21203/rs.3.rs-6735294/v1. Res Sq. 2025. PMID: 40585236 Free PMC article. Preprint.
-
An Open-Source Deep Learning-Based GUI Toolbox for Automated Auditory Brainstem Response Analyses (ABRA).bioRxiv [Preprint]. 2025 Apr 2:2024.06.20.599815. doi: 10.1101/2024.06.20.599815. bioRxiv. 2025. PMID: 38948763 Free PMC article. Preprint.
-
Cochlear Amyloid-β42 Accumulation Drives Progressive Auditory Neuropathy in 5XFAD Mice: A Potential Biomarker for Early Alzheimer's Disease.Res Sq [Preprint]. 2025 Apr 21:rs.3.rs-6431143. doi: 10.21203/rs.3.rs-6431143/v1. Res Sq. 2025. PMID: 40313736 Free PMC article. Preprint.
-
Exploring biomarkers for noise-induced hearing loss through mitochondrial DNA methylation analysis.Front Pharmacol. 2025 Jul 4;16:1561791. doi: 10.3389/fphar.2025.1561791. eCollection 2025. Front Pharmacol. 2025. PMID: 40689209 Free PMC article.
-
Roles of Sirtuins in Hearing Protection.Pharmaceuticals (Basel). 2024 Jul 28;17(8):998. doi: 10.3390/ph17080998. Pharmaceuticals (Basel). 2024. PMID: 39204103 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
