Biology of Stress Responses in Aging
- PMID: 38500537
- PMCID: PMC10947073
- DOI: 10.59368/agingbio.20230001
Biology of Stress Responses in Aging
Abstract
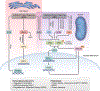
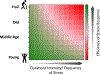
On April 28th, 2022, a group of scientific leaders gathered virtually to discuss molecular and cellular mechanisms of responses to stress. Conditions of acute, high-intensity stress are well documented to induce a series of adaptive responses that aim to promote survival until the stress has dissipated and then guide recovery. However, high-intensity or persistent stress that goes beyond the cell's compensatory capacity are countered with resilience strategies that are not completely understood. These adaptative strategies, which are an essential component of the study of aging biology, were the theme of the meeting. Specific topics discussed included mechanisms of proteostasis, such as the unfolded protein response (UPR) and the integrated stress response (ISR), as well as mitochondrial stress and lysosomal stress responses. Attention was also given to regulatory mechanisms and associated biological processes linked to age-related conditions, such as muscle loss and regeneration, cancer, senescence, sleep quality, and degenerative disease, with a general focus on the relevance of stress responses to frailty. We summarize the concepts and potential future directions that emerged from the discussion and highlight their relevance to the study of aging and age-related chronic diseases.
Figures

References
-
- Galluzzi L, Yamazaki T & Kroemer G Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol 19, 731–745 (2018). - PubMed
-
- Hetz C The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol 13, 89–102 (2012). - PubMed
-
- Maurel M, Chevet E, Tavernier J & Gerlo S Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci 39, 245–254 (2014). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
