Developing a novel magnetic organic polymer for selective extraction and determination of 16 macrolides in water and honey samples
- PMID: 38500629
- PMCID: PMC10945740
- DOI: 10.1039/d4ra00496e
Developing a novel magnetic organic polymer for selective extraction and determination of 16 macrolides in water and honey samples
Abstract
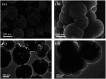
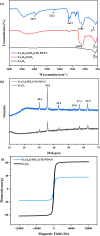
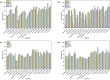
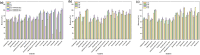
A novel magnetic organic polymer Fe3O4@SiO2@Tb-PDAN was designed and synthesized, which was used as an adsorbent for magnetic solid-phase extraction (MSPE) of 16 macrolides (MALs) in water and honey. The synthesized adsorbent was characterized using techniques including scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR) and X-ray diffraction (XRD). Then several parameters of the extraction process were further optimized. Under the optimized conditions, an MSPE-LC-MS/MS method was established for extraction and determination of 16 MALs, which showed good linearity (r ≥ 0.999), low limits of detection (0.001-0.012 μg L-1 for water and 0.001-0.367 μg kg-1 for honey) and satisfactory recoveries (70.02-118.91%) with the relative standard deviations (RSDs) lower than 10.0%. This established method was then successfully applied to detect MALs in real samples, which suggested that Fe3O4@SiO2@Tb-PDAN was a potential magnetic adsorbent for efficient extraction and analysis of MALs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Fe3O4@SiO2-BD-DADB-COF is proposed as a novel magnetic covalent organic framework for the determination and extraction of 15 macrolide antibiotics in water and honey.RSC Adv. 2025 Mar 17;15(11):8111-8120. doi: 10.1039/d5ra00080g. eCollection 2025 Mar 17. RSC Adv. 2025. PMID: 40103973 Free PMC article.
-
[Determination of four fungicides in water by magnetic solid phase extraction-ultrahigh performance liquid chromatography-tandem mass spectrometry using covalent organic framework material].Se Pu. 2022 Nov;40(11):988-997. doi: 10.3724/SP.J.1123.2022.08023. Se Pu. 2022. PMID: 36351807 Free PMC article. Chinese.
-
[Determination of 10 fluoroquinolones residues in aquatic products by accelerated solvent extraction, magnetic solid-phase extraction, and high-performance liquid chromatography-tandem mass spectrometry].Se Pu. 2020 Dec 8;38(12):1413-1422. doi: 10.3724/SP.J.1123.2020.05002. Se Pu. 2020. PMID: 34213256 Chinese.
-
Magnetic solid-phase extraction based on GO/Fe3O4 coupled with UPLC-MS/MS for determining nitroimidazoles and their metabolites in honey.Talanta. 2023 Mar 1;254:124181. doi: 10.1016/j.talanta.2022.124181. Epub 2022 Dec 8. Talanta. 2023. PMID: 36512971
-
[Determination of three diphenyl ether herbicides in rice by magnetic solid phase extraction using Fe3O4@MOF-808 coupled with high performance liquid chromatography].Se Pu. 2021 Mar;39(3):316-323. doi: 10.3724/SP.J.1123.2020.06007. Se Pu. 2021. PMID: 34227312 Free PMC article. Chinese.
Cited by
-
[Recent progress in magnetic covalent organic framework materials for the enrichment and detection of typical organic pollutants].Se Pu. 2025 Feb;43(2):107-119. doi: 10.3724/SP.J.1123.2024.07020. Se Pu. 2025. PMID: 39844701 Free PMC article. Chinese.
-
Fe3O4@SiO2-BD-DADB-COF is proposed as a novel magnetic covalent organic framework for the determination and extraction of 15 macrolide antibiotics in water and honey.RSC Adv. 2025 Mar 17;15(11):8111-8120. doi: 10.1039/d5ra00080g. eCollection 2025 Mar 17. RSC Adv. 2025. PMID: 40103973 Free PMC article.
References
LinkOut - more resources
Full Text Sources

