High-resolution 3D ultrastructural analysis of developing mouse neocortex reveals long slender processes of endothelial cells that enter neural cells
- PMID: 38500687
- PMCID: PMC10945550
- DOI: 10.3389/fcell.2024.1344734
High-resolution 3D ultrastructural analysis of developing mouse neocortex reveals long slender processes of endothelial cells that enter neural cells
Abstract
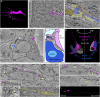
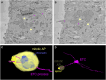
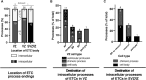
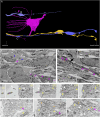
The development of the neocortex involves an interplay between neural cells and the vasculature. However, little is known about this interplay at the ultrastructural level. To gain a 3D insight into the ultrastructure of the developing neocortex, we have analyzed the embryonic mouse neocortex by serial block-face scanning electron microscopy (SBF-SEM). In this study, we report a first set of findings that focus on the interaction of blood vessels, notably endothelial tip cells (ETCs), and the neural cells in this tissue. A key observation was that the processes of ETCs, located either in the ventricular zone (VZ) or subventricular zone (SVZ)/intermediate zone (IZ), can enter, traverse the cytoplasm, and even exit via deep plasma membrane invaginations of the host cells, including apical progenitors (APs), basal progenitors (BPs), and newborn neurons. More than half of the ETC processes were found to enter the neural cells. Striking examples of this ETC process "invasion" were (i) protrusions of apical progenitors or newborn basal progenitors into the ventricular lumen that contained an ETC process inside and (ii) ETC process-containing protrusions of neurons that penetrated other neurons. Our observations reveal a - so far unknown - complexity of the ETC-neural cell interaction.
Keywords: apical progenitors; basal progenitors; cortical development; endothelial tip cell; neurogenesis; radial glial cells; sprouting angiogenesis; volume electron microscopy.
Copyright © 2024 Wilsch-Bräuninger, Peters and Huttner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors.J Neurosci. 2011 Mar 9;31(10):3683-95. doi: 10.1523/JNEUROSCI.4773-10.2011. J Neurosci. 2011. PMID: 21389223 Free PMC article.
-
Conditional Inactivation of Pen-2 in the Developing Neocortex Leads to Rapid Switch of Apical Progenitors to Basal Progenitors.J Neurosci. 2019 Mar 20;39(12):2195-2207. doi: 10.1523/JNEUROSCI.2523-18.2019. Epub 2019 Jan 28. J Neurosci. 2019. PMID: 30692224 Free PMC article.
-
Differences of migratory behavior between direct progeny of apical progenitors and basal progenitors in the developing cerebral cortex.Cereb Cortex. 2009 Sep;19(9):2092-105. doi: 10.1093/cercor/bhn227. Epub 2009 Jan 15. Cereb Cortex. 2009. PMID: 19150920
-
The SVZ stem cell niche-components, functions, and in vitro modelling.Front Cell Dev Biol. 2023 Dec 22;11:1332901. doi: 10.3389/fcell.2023.1332901. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38188021 Free PMC article. Review.
-
Cytoarchitecture of mouse and human subventricular zone in developing cerebral neocortex.Exp Brain Res. 2012 Jan;216(2):161-8. doi: 10.1007/s00221-011-2933-3. Epub 2011 Nov 13. Exp Brain Res. 2012. PMID: 22080150 Free PMC article. Review.
Cited by
-
The retina's neurovascular unit: Müller glial sheaths and neuronal contacts.bioRxiv [Preprint]. 2024 May 1:2024.04.30.591885. doi: 10.1101/2024.04.30.591885. bioRxiv. 2024. PMID: 38903067 Free PMC article. Preprint.
-
Layer-specific anatomical and physiological features of the retina's neurovascular unit.Curr Biol. 2025 Jan 6;35(1):109-120.e4. doi: 10.1016/j.cub.2024.11.023. Epub 2024 Dec 16. Curr Biol. 2025. PMID: 39689705 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

