Benefit, recurrence pattern, and toxicity to adjuvant anti-PD-1 monotherapy varies by ethnicity and melanoma subtype: An international multicenter cohort study
- PMID: 38500872
- PMCID: PMC10945245
- DOI: 10.1016/j.jdin.2023.11.014
Benefit, recurrence pattern, and toxicity to adjuvant anti-PD-1 monotherapy varies by ethnicity and melanoma subtype: An international multicenter cohort study
Abstract
Background: Anti-Program-Death-1 (PD-1) is a standard adjuvant therapy for patients with resected melanoma. We hypothesized that there are discrepancies in survival, recurrence pattern and toxicity to adjuvant PD-1 between different ethnicities and melanoma subtypes.
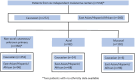
Objective: We performed a multicenter cohort study incorporating 6 independent institutions in Australia, China, Japan, and the United States. The primary outcomes were recurrence free survival (RFS) and overall survival (OS). Secondary outcomes were disease recurrence patterns and toxicities.
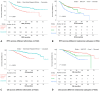
Results: In total 534 patients were included. East-Asian/Hispanic/African reported significantly poorer RFS/OS. Nonacral cutaneous or melanoma of unknown primary reported the best RFS/OS, followed by acral, and mucosal was the poorest. Within the nonacral cutaneous or melanoma of unknown primary subtypes, East-Asian/Hispanic/African reported significantly poorer RFS/OS than Caucasian. In the multivariate analysis incorporating ethnicity/melanoma-subtype/age/sex/stage/lactate dehydrogenase/BRAF (v-Raf murine sarcoma viral oncogene homolog B)-mutation/adjuvant radiotherapy, East-Asian/Hispanic/African had independently significantly poorer outcomes (RFS: HR, 1.71; 95% CI, 1.19-2.44 and OS: HR, 2.34; 95% CI, 1.39-3.95), as was mucosal subtype (RFS: HR, 3.25; 95% CI, 2.04-5.17 and OS: HR, 3.20; 95% CI, 1.68-6.08). Mucosal melanoma was an independent risk factor for distant metastasis, especially liver metastasis. East-Asian/Hispanic/African had significantly lower incidence of gastrointestinal/musculoskeletal/respiratory/other-rare-type-toxicities; but higher incidences of liver toxicities.
Limitations: A retrospective study.
Conclusions: Ethnicity and melanoma subtype are associated with survival and recurrence pattern in melanoma patients treated with adjuvant anti-PD-1. Toxicity profile differs by ethnicity and may require a precision toxicity surveillance strategy.
Keywords: adjuvant PD-1; efficacy; ethnicity; melanoma subtype; toxicity.
© 2024 Published by Elsevier Inc. on behalf of the American Academy of Dermatology, Inc.
Conflict of interest statement
Dr Yamazaki has received honoraria from Ono pharmaceutical, 10.13039/100002491Bristol-Myers Squibb, MSD, 10.13039/100004336Novartis, and Maruho; has received research grant from Ono pharmaceutical, 10.13039/100002491Bristol-Myers Squibb, 10.13039/100004336Novartis, and 10.13039/100002429Amgen. Dr Ogata has received honoraria from MSD, Novartis, Ono pharmaceutical, and Bristol-Myers Squibb. Dr Nakamura serves on a scientific advisory board and educational steering committee for 10.13039/100004336Novartis, has received honoraria from BMS, Kyowa-Kirin, Maruho, MSD, 10.13039/100004336Novartis, Ono Pharma, and Tanabe-Mitsubishi Pharma, and received institutional grants from Kaken Pharma, Ono, Pola Pharma, and Torii. Dr Namikawa has received honoraria from Ono pharmaceutical, Novartis, Bristol-Myers Squibb, and MSD; serves as an advisory role for Novartis and MSD. Dr Guo serves as consultant or is on advisory boards for MSD, Roche, Pfizer, Bayer, Novartis, Simcere Pharmaceutical Group, Shanghai Junshi Biosciences, and Oriengene. Dr Flaherty serves on the Board of Directors of Clovis Oncology, Strata Oncology, Vivid Biosciences, and Scorpion Therapeutics;Scientific Advisory Boards of PIC Therapeutics, Apricity, Tvardi, ALX Oncology, xCures, Monopteros, Vibliome, and Soley Therapeutics; consultant to Takeda, Novartis and Transcode Therapeutics. Dr Long serves as a consultant advisor for Agenus, Amgen, Array Biopharma, AstraZeneca, Boehringer Ingelheim, BMS, Evaxion, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics, MSD, Novartis, OncoSec, PHMR Ltd, Pierre-Fabre, Provectus, Qbiotics, Regeneron. Dr Menzies serves as a consultant for BMS, MSD, Novartis, Roche, Pierre-Fabre and QBiotics. Dr D.B. Johnson has served on advisory boards or as a consultant for BMS, Catalyst Biopharma, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer, Targovax, and Teiko, has received research funding from BMS and Incyte. Dr Sullivan serves as consultant for Amgen, Asana Biosciences, BMS, Merck, Novartis, Array BioPharma, Compugen, and Replimune; he receives research support from Amgen and Merck. Dr Boland has a sponsored research agreements with Takeda Oncology, Palleon Pharmaceuticals, InterVenn Biosciences, and Olink Proteomics; serves as a consultant for 10.13039/100004334Merck, InterVenn Biosciences, and Ankyra Therapeutics; served as a speaker for Novartis; and served on a scientific advisory board and steering committee for Nektar Therapeutics. Dr Si has received speakers’ honoraria from MSD, Roche, Novartis, Shanghai Junshi Biosciences and Oriengene. Drs Bai, Gerstberger, Park, Umeda and Authors Lawless, Czapla, Jung, R. Johnson, Li have no conflicts of interest to declare.
Figures

Similar articles
-
Benefit and toxicity of programmed death-1 blockade vary by ethnicity in patients with advanced melanoma: an international multicentre observational study.Br J Dermatol. 2022 Sep;187(3):401-410. doi: 10.1111/bjd.21241. Epub 2022 May 20. Br J Dermatol. 2022. PMID: 35293617
-
Dabrafenib plus trametinib versus anti-PD-1 monotherapy as adjuvant therapy in BRAF V600-mutant stage III melanoma after definitive surgery: a multicenter, retrospective cohort study.EClinicalMedicine. 2023 Oct 31;65:102290. doi: 10.1016/j.eclinm.2023.102290. eCollection 2023 Nov. EClinicalMedicine. 2023. PMID: 37965433 Free PMC article.
-
Comparative analysis of adjuvant therapy for stage III BRAF-mut melanoma: A real-world retrospective study from single center in China.Cancer Med. 2023 May;12(10):11475-11482. doi: 10.1002/cam4.5866. Epub 2023 Apr 4. Cancer Med. 2023. PMID: 37016119 Free PMC article.
-
Adjuvant Therapy of High-Risk (Stages IIC-IV) Malignant Melanoma in the Post Interferon-Alpha Era: A Systematic Review and Meta-Analysis.Front Oncol. 2021 Feb 18;10:637161. doi: 10.3389/fonc.2020.637161. eCollection 2020. Front Oncol. 2021. PMID: 33680957 Free PMC article.
-
Adjuvant Therapy in Acral Melanoma: A Systematic Review.Clin Cosmet Investig Dermatol. 2024 Sep 25;17:2141-2150. doi: 10.2147/CCID.S477155. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 39345988 Free PMC article. Review.
Cited by
-
The Evaluation of Immune Checkpoint Inhibitors and BRAF/MEK Inhibitors in Different Therapy Lines for Metastatic Melanoma: A Retrospective Study.J Clin Med. 2024 Sep 19;13(18):5560. doi: 10.3390/jcm13185560. J Clin Med. 2024. PMID: 39337055 Free PMC article.
-
Three-Year Analysis of Adjuvant Therapy in Postoperative Melanoma including Acral and Mucosal Subtypes.Cancers (Basel). 2024 Aug 2;16(15):2755. doi: 10.3390/cancers16152755. Cancers (Basel). 2024. PMID: 39123482 Free PMC article.
-
Poor efficacy of anti PD-1 antibody based immunotherapy in patients with acral melanoma: results from the Spanish Melanoma Group (GEM) registry.Clin Transl Oncol. 2025 Aug 21. doi: 10.1007/s12094-025-04018-5. Online ahead of print. Clin Transl Oncol. 2025. PMID: 40841506
-
Adjuvant Anti-PD-1 Monotherapy Versus Observation for Stage III Acral Melanoma of the Sole: A Multicenter Retrospective Study in Japanese Patients.JCO Glob Oncol. 2025 Apr;11:e2400644. doi: 10.1200/GO-24-00644. Epub 2025 Apr 4. JCO Glob Oncol. 2025. PMID: 40184568 Free PMC article.
-
Effectiveness of immune checkpoint inhibitors and other treatment modalities in patients with advanced mucosal melanomas: a systematic review and individual patient data meta-analysis.EClinicalMedicine. 2024 Oct 4;77:102870. doi: 10.1016/j.eclinm.2024.102870. eCollection 2024 Nov. EClinicalMedicine. 2024. PMID: 39416390 Free PMC article.
References
-
- Grossmann K.F., Othus M., Patel S.P., et al. Adjuvant Pembrolizumab versus IFNalpha2b or ipilimumab in Resected High-Risk Melanoma. Cancer Discov. 2022;12(3):644–653. doi: 10.1158/2159-8290.CD-21-1141. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

