Disrupted propionate metabolism evokes transcriptional changes in the heart by increasing histone acetylation and propionylation
- PMID: 38500966
- PMCID: PMC7615744
- DOI: 10.1038/s44161-023-00365-0
Disrupted propionate metabolism evokes transcriptional changes in the heart by increasing histone acetylation and propionylation
Abstract
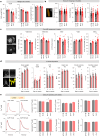
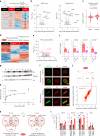
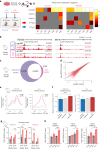
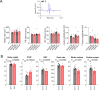
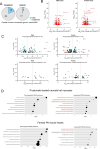
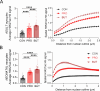
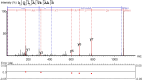
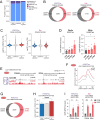
Propiogenic substrates and gut bacteria produce propionate, a post-translational protein modifier. In this study, we used a mouse model of propionic acidaemia (PA) to study how disturbances to propionate metabolism result in histone modifications and changes to gene expression that affect cardiac function. Plasma propionate surrogates were raised in PA mice, but female hearts manifested more profound changes in acyl-CoAs, histone propionylation and acetylation, and transcription. These resulted in moderate diastolic dysfunction with raised diastolic Ca2+, expanded end-systolic ventricular volume and reduced stroke volume. Propionate was traced to histone H3 propionylation and caused increased acetylation genome-wide, including at promoters of Pde9a and Mme, genes related to contractile dysfunction through downscaled cGMP signaling. The less severe phenotype in male hearts correlated with β-alanine buildup. Raising β-alanine in cultured myocytes treated with propionate reduced propionyl-CoA levels, indicating a mechanistic relationship. Thus, we linked perturbed propionate metabolism to epigenetic changes that impact cardiac function.
Conflict of interest statement
Competing interests N.T.C. and T.A.M. are shareholders in and consultants for Dark Blue Therapeutics, Ltd. The remaining authors declare no competing interests.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
