Ursolic acid inhibits glioblastoma through suppressing TGFβ-mediated epithelial-mesenchymal transition (EMT) and angiogenesis
- PMID: 38501006
- PMCID: PMC10945258
- DOI: 10.1016/j.heliyon.2024.e27722
Ursolic acid inhibits glioblastoma through suppressing TGFβ-mediated epithelial-mesenchymal transition (EMT) and angiogenesis
Abstract
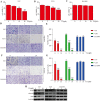
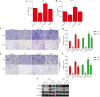
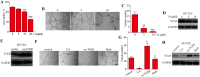
Found in many fruits and plants, Ursolic acid (UA), a pentacyclic triterpene that occurs naturally, is recognized for its anti-cancer effects, especially in combating glioblastoma. However, the intricate molecular mechanisms underpinning its anti-tumor actions are still not fully understood, despite the recognition of these effects. By examining the functions of epithelial-mesenchymal transition (EMT) and angiogenesis, crucial for glioblastoma progression, and their regulation through Transforming Growth Factor Beta (TGFβ) - a key marker for glioblastoma, our research aims to fill this knowledge gap. This study explores how ursolic acid can block the progression of glioblastoma by precisely targeting TGFβ-triggered EMT and angiogenesis. The findings show that UA successfully blocks the spread, movement, and invasion of glioblastoma cells. Accompanying this, there is a significant reduction in the expression of TGFβ and crucial EMT indicators like snail and vimentin. Furthermore, UA shows a reduction in angiogenesis that depends on the dosage, highlighted by decreased vascular endothelial growth factor (VEGF) in human umbilical vein endothelial cells (HUVECs). Interestingly, increased TGFβ expression in U87 and U251 glioblastoma cell lines was found to weaken UA's anti-tumor properties, shedding more light on TGFβ's critical function in glioblastoma's pathology. Supporting these laboratory results, UA also showed considerable inhibition of tumor growth in a glioblastoma xenograft mouse model. Overall, our research emphasizes Ursolic acid's promise as a new treatment for glioblastoma and clarifies its action mechanism, mainly by inhibiting TGFβ signaling and thereby EMT and angiogenesis.
Keywords: Angiogenesis; Epithelial-mesenchymal transition (EMT); Glioblastoma; TGFβ; Ursolic acid.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Di Carlo D.T., et al. Multiple high-grade gliomas: epidemiology, management, and outcome. A systematic review and meta-analysis. Neurosurg. Rev. 2019;42(2):263–275. - PubMed
-
- Majc B., et al. Epithelial-to-mesenchymal transition as the driver of changing carcinoma and glioblastoma microenvironment. Biochim. Biophys. Acta, Mol. Cell Res. 2020;1867(10) - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

