Hypoxia-inducible factor-2α promotes fibrosis in non-alcoholic fatty liver disease by enhancing glutamine catabolism and inhibiting yes-associated protein phosphorylation in hepatic stellate cells
- PMID: 38501098
- PMCID: PMC10946064
- DOI: 10.3389/fendo.2024.1344971
Hypoxia-inducible factor-2α promotes fibrosis in non-alcoholic fatty liver disease by enhancing glutamine catabolism and inhibiting yes-associated protein phosphorylation in hepatic stellate cells
Abstract
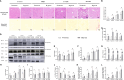
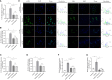
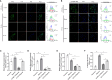
Non-alcoholic fatty liver disease (NAFLD) has a high global prevalence and affects approximately one-third of adults, owing to high-fat dietary habits and a sedentary lifestyle. The role of hypoxia-inducible factor 2α (HIF-2α) in NAFLD progression remains unknown. This study aimed to investigate the effects of chronic hypoxia on NAFLD progression by examining the role of hypoxia-inducible factor 2α (HIF-2α) activation and that of hepatic stellate cell (HSC)-derived myofibroblasts through glutaminolysis. We hypothesised that hypoxia exacerbates NAFLD by promoting HIF-2α upregulation and inhibiting phosphorylated yes-associated protein (YAP), and that increasing YAP expression enhances HSC-derived myofibroblasts. We studied patients with NAFLD living at high altitudes, as well as animal models and cultured cells. The results revealed significant increases in HSC-derived myofibroblasts and collagen accumulation caused by HIF-2α and YAP upregulation, both in patients and in a mouse model for hypoxia and NAFLD. HIF-2α and HIF-2α-dependent YAP downregulation reduced HSC activation and myofibroblast levels in persistent chronic hypoxia. Furthermore, hypoxia-induced HIF-2α upregulation promoted YAP and inhibited YAP phosphorylation, leading to glutaminase 1 (GLS1), SLC38A1, α-SMA, and Collagen-1 overexpression. Additionally, hypoxia restored mitochondrial adenosine triphosphate production and reactive oxygen species (ROS) overproduction. Thus, chronic hypoxia-induced HIF-2α activation enhances fibrosis and NAFLD progression by restoring mitochondrial ROS production and glutaminase-1-induced glutaminolysis, which is mediated through the inhibition of YAP phosphorylation and increased YAP nuclear translocation. In summary, HIF-2α plays a pivotal role in NAFLD progression during chronic hypoxia.
Keywords: HIF-2α; NAFLD/NASH; YAP/p-YAP; glutaminolysis; hepatic stellate cells-derived myofibroblasts.
Copyright © 2024 Yan, Cai, Zhou, Bao, Bai and Ge.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

