Cryo-electron microscopy-based drug design
- PMID: 38501110
- PMCID: PMC10945328
- DOI: 10.3389/fmolb.2024.1342179
Cryo-electron microscopy-based drug design
Abstract
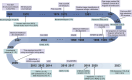
Structure-based drug design (SBDD) has gained popularity owing to its ability to develop more potent drugs compared to conventional drug-discovery methods. The success of SBDD relies heavily on obtaining the three-dimensional structures of drug targets. X-ray crystallography is the primary method used for solving structures and aiding the SBDD workflow; however, it is not suitable for all targets. With the resolution revolution, enabling routine high-resolution reconstruction of structures, cryogenic electron microscopy (cryo-EM) has emerged as a promising alternative and has attracted increasing attention in SBDD. Cryo-EM offers various advantages over X-ray crystallography and can potentially replace X-ray crystallography in SBDD. To fully utilize cryo-EM in drug discovery, understanding the strengths and weaknesses of this technique and noting the key advancements in the field are crucial. This review provides an overview of the general workflow of cryo-EM in SBDD and highlights technical innovations that enable its application in drug design. Furthermore, the most recent achievements in the cryo-EM methodology for drug discovery are discussed, demonstrating the potential of this technique for advancing drug development. By understanding the capabilities and advancements of cryo-EM, researchers can leverage the benefits of designing more effective drugs. This review concludes with a discussion of the future perspectives of cryo-EM-based SBDD, emphasizing the role of this technique in driving innovations in drug discovery and development. The integration of cryo-EM into the drug design process holds great promise for accelerating the discovery of new and improved therapeutic agents to combat various diseases.
Keywords: cryo-electron microscopy; drug development; high-resolution; single particle analysis; structure-based drug design.
Copyright © 2024 Cebi, Lee, Subramani, Bak, Oh and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Structure determination of GPCRs: cryo-EM compared with X-ray crystallography.Biochem Soc Trans. 2021 Nov 1;49(5):2345-2355. doi: 10.1042/BST20210431. Biochem Soc Trans. 2021. PMID: 34581758 Free PMC article. Review.
-
Dynamics-based drug discovery by time-resolved cryo-EM.Curr Opin Struct Biol. 2025 Apr;91:103001. doi: 10.1016/j.sbi.2025.103001. Epub 2025 Feb 21. Curr Opin Struct Biol. 2025. PMID: 39985947 Review.
-
Cryo-EM in drug discovery: achievements, limitations and prospects.Nat Rev Drug Discov. 2018 Jul;17(7):471-492. doi: 10.1038/nrd.2018.77. Epub 2018 Jun 8. Nat Rev Drug Discov. 2018. PMID: 29880918 Review.
-
Progress in spatial resolution of structural analysis by cryo-EM.Microscopy (Oxf). 2023 Apr 6;72(2):135-143. doi: 10.1093/jmicro/dfac053. Microscopy (Oxf). 2023. PMID: 36269102 Review.
-
Developments, applications, and prospects of cryo-electron microscopy.Protein Sci. 2020 Apr;29(4):872-882. doi: 10.1002/pro.3805. Epub 2019 Dec 26. Protein Sci. 2020. PMID: 31854478 Free PMC article. Review.
Cited by
-
Importance of Computer-aided Drug Design in Modern Pharmaceutical Research.Curr Drug Discov Technol. 2025;22(3):e15701638361318. doi: 10.2174/0115701638361318241230073123. Curr Drug Discov Technol. 2025. PMID: 39810447 Review.
-
A goldilocks computational protocol for inhibitor discovery targeting DNA damage responses including replication-repair functions.Front Mol Biosci. 2024 Nov 28;11:1442267. doi: 10.3389/fmolb.2024.1442267. eCollection 2024. Front Mol Biosci. 2024. PMID: 39669672 Free PMC article.
-
Practical Guide for Implementing Cryogenic Electron Microscopy Structure Determination in Dermatology Research.J Invest Dermatol. 2025 Jan;145(1):22-31. doi: 10.1016/j.jid.2024.10.594. Epub 2024 Nov 26. J Invest Dermatol. 2025. PMID: 39601740 Review.
-
The next generation of drug resistant tuberculosis drug design.Future Med Chem. 2025 Feb;17(4):385-387. doi: 10.1080/17568919.2025.2453406. Epub 2025 Jan 15. Future Med Chem. 2025. PMID: 39814693 No abstract available.
References
Publication types
LinkOut - more resources
Full Text Sources

