Cross-species modeling of muscular dystrophy in Caenorhabditis elegans using patient-derived extracellular vesicles
- PMID: 38501170
- PMCID: PMC11007864
- DOI: 10.1242/dmm.050412
Cross-species modeling of muscular dystrophy in Caenorhabditis elegans using patient-derived extracellular vesicles
Abstract
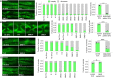
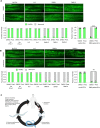
Reliable disease models are critical for medicine advancement. Here, we established a versatile human disease model system using patient-derived extracellular vesicles (EVs), which transfer a pathology-inducing cargo from a patient to a recipient naïve model organism. As a proof of principle, we applied EVs from the serum of patients with muscular dystrophy to Caenorhabditis elegans and demonstrated their capability to induce a spectrum of muscle pathologies, including lifespan shortening and robust impairment of muscle organization and function. This demonstrates that patient-derived EVs can deliver disease-relevant pathologies between species and can be exploited for establishing novel and personalized models of human disease. Such models can potentially be used for disease diagnosis, prognosis, analyzing treatment responses, drug screening and identification of the disease-transmitting cargo of patient-derived EVs and their cellular targets. This system complements traditional genetic disease models and enables modeling of multifactorial diseases and of those not yet associated with specific genetic mutations.
Keywords: Caenorhabditis elegans; Disease modeling; Duchenne muscular dystrophy; Extracellular vesicles.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
Dauer larva-derived extracellular vesicles extend the life of Caenorhabditis elegans.Biogerontology. 2023 Aug;24(4):581-592. doi: 10.1007/s10522-023-10030-5. Epub 2023 Apr 13. Biogerontology. 2023. PMID: 37052773 Free PMC article.
-
Caenorhabditis elegans as a Model System for Duchenne Muscular Dystrophy.Int J Mol Sci. 2021 May 5;22(9):4891. doi: 10.3390/ijms22094891. Int J Mol Sci. 2021. PMID: 34063069 Free PMC article. Review.
-
Cilia-Derived Extracellular Vesicles in Caenorhabditis Elegans: In Vivo Imaging and Quantification of Extracellular Vesicle Release and Capture.Methods Mol Biol. 2023;2668:277-299. doi: 10.1007/978-1-0716-3203-1_19. Methods Mol Biol. 2023. PMID: 37140803
-
Prednisone reduces muscle degeneration in dystrophin-deficient Caenorhabditis elegans.Neuromuscul Disord. 2004 Jun;14(6):365-70. doi: 10.1016/j.nmd.2004.02.011. Neuromuscul Disord. 2004. PMID: 15145337
-
Muscular dystrophy: the worm turns to genetic disease.Curr Biol. 2000 Nov 2;10(21):R795-7. doi: 10.1016/s0960-9822(00)00768-5. Curr Biol. 2000. PMID: 11084353 Review.
Cited by
-
Multiscale brain modeling: bridging microscopic and macroscopic brain dynamics for clinical and technological applications.Front Cell Neurosci. 2025 Feb 19;19:1537462. doi: 10.3389/fncel.2025.1537462. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40046848 Free PMC article. Review.
References
-
- Aartsma-Rus, A., Straub, V., Hemmings, R., Haas, M., Schlosser-Weber, G., Stoyanova-Beninska, V., Mercuri, E., Muntoni, F., Sepodes, B., Vroom, E.et al. (2017). Development of exon skipping therapies for duchenne muscular dystrophy: a critical review and a perspective on the outstanding issues. Nucleic Acid Ther. 27, 251-259. 10.1089/nat.2017.0682 - DOI - PMC - PubMed
-
- Azkona, G. and Sanchez-Pernaute, R. (2022). Mice in translational neuroscience: what R we doing? Prog. Neurobiol. 217, 102330. - PubMed
-
- Baldridge, D., Wangler, M. F., Bowman, A. N., Yamamoto, S., Schedl, T., Pak, S. C., Postlethwait, J. H., Shin, J., Solnica-Krezel, L., Bellen, H. J.et al. (2021). Model organisms contribute to diagnosis and discovery in the undiagnosed diseases network: current state and a future vision. Orphanet J. Rare Dis. 16, 206. 10.1186/s13023-021-01839-9 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

