Targeting metabolism to improve CAR-T cells therapeutic efficacy
- PMID: 38501360
- PMCID: PMC11046027
- DOI: 10.1097/CM9.0000000000003046
Targeting metabolism to improve CAR-T cells therapeutic efficacy
Abstract
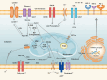
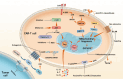
Chimeric antigen receptor T (CAR-T) cell therapy achieved advanced progress in the treatment of hematological tumors. However, the application of CAR-T cell therapy for solid tumors still faces many challenges. Competition with tumor cells for metabolic resources in an already nutrient-poor tumor microenvironment is a major contributing cause to CAR-T cell therapy's low effectiveness. Abnormal metabolic processes are now acknowledged to shape the tumor microenvironment, which is characterized by increased interstitial fluid pressure, low pH level, hypoxia, accumulation of immunosuppressive metabolites, and mitochondrial dysfunction. These factors are important contributors to restriction of T cell proliferation, cytokine release, and suppression of tumor cell-killing ability. This review provides an overview of how different metabolites regulate T cell activity, analyzes the current dilemmas, and proposes key strategies to reestablish the CAR-T cell therapy's effectiveness through targeting metabolism, with the aim of providing new strategies to surmount the obstacle in the way of solid tumor CAR-T cell treatment.
Copyright © 2024 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures
References
-
- Labanieh L, Mackall CL. CAR immune cells: design principles, resistance and the next generation. Nature 2023;614:635–648. doi: 10.1038/s41586-023-05707-3. - PubMed
-
- The NMPA approved the Naki Orensei Injection from He Yuan Biotechnology (Tianjin) Co. for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia in adults. Available from: https://www.nmpa.gov.cn/zhuanti/cxylqx/cxypxx/20231108092415187.html. [Accessed on December 2, 2023].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

