Mechanosensitive channel MscL gating transitions coupling with constriction point shift
- PMID: 38501596
- PMCID: PMC10949393
- DOI: 10.1002/pro.4965
Mechanosensitive channel MscL gating transitions coupling with constriction point shift
Abstract
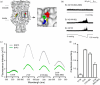
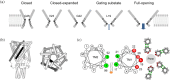
The mechanosensitive channel of large conductance (MscL) acts as an "emergency release valve" that protects bacterial cells from acute hypoosmotic stress, and it serves as a paradigm for studying the mechanism underlying the transduction of mechanical forces. MscL gating is proposed to initiate with an expansion without opening, followed by subsequent pore opening via a number of intermediate substates, and ends in a full opening. However, the details of gating process are still largely unknown. Using in vivo viability assay, single channel patch clamp recording, cysteine cross-linking, and tryptophan fluorescence quenching approach, we identified and characterized MscL mutants with different occupancies of constriction region in the pore domain. The results demonstrated the shifts of constriction point along the gating pathway towards cytoplasic side from residue G26, though G22, to L19 upon gating, indicating the closed-expanded transitions coupling of the expansion of tightly packed hydrophobic constriction region to conduct the initial ion permeation in response to the membrane tension. Furthermore, these transitions were regulated by the hydrophobic and lipidic interaction with the constricting "hot spots". Our data reveal a new resolution of the transitions from the closed to the opening substate of MscL, providing insights into the gating mechanisms of MscL.
Keywords: MscL; constriction point; gating pore; gating substate; gating transitions; mechanosensitive channel; protien-lipid interaction.
© 2024 The Protein Society.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
On the role of individual subunits in MscL gating: "all for one, one for all?".FASEB J. 2013 Mar;27(3):882-92. doi: 10.1096/fj.12-214361. Epub 2012 Nov 27. FASEB J. 2013. PMID: 23193173
-
An in vivo assay identifies changes in residue accessibility on mechanosensitive channel gating.Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10161-5. doi: 10.1073/pnas.0402040101. Epub 2004 Jun 28. Proc Natl Acad Sci U S A. 2004. PMID: 15226501 Free PMC article.
-
An open-pore structure of the mechanosensitive channel MscL derived by determining transmembrane domain interactions upon gating.FASEB J. 2009 Jul;23(7):2197-204. doi: 10.1096/fj.09-129296. Epub 2009 Mar 4. FASEB J. 2009. PMID: 19261722 Free PMC article.
-
Mechanosensitive channels in bacteria as membrane tension reporters.FASEB J. 1999;13 Suppl:S55-61. doi: 10.1096/fasebj.13.9001.s55. FASEB J. 1999. PMID: 10352145 Review.
-
MscL: channeling membrane tension.Pflugers Arch. 2015 Jan;467(1):15-25. doi: 10.1007/s00424-014-1535-x. Epub 2014 May 27. Pflugers Arch. 2015. PMID: 24859800 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

