Fatty acids of Helicobacter pylori lipoproteins CagT and Lpp20
- PMID: 38501821
- PMCID: PMC11064636
- DOI: 10.1128/spectrum.00470-24
Fatty acids of Helicobacter pylori lipoproteins CagT and Lpp20
Abstract
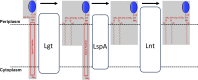
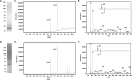
Bacterial lipoproteins are post-translationally modified by the addition of acyl chains that anchor the protein to bacterial membranes. This modification includes two ester-linked and one amide-linked acyl chain on lipoproteins from Gram-negative bacteria. Helicobacter pylori lipoproteins have important functions in pathogenesis (including delivering the CagA oncoprotein to mammalian cells) and are recognized by host innate and adaptive immune systems. The number and variety of acyl chains on lipoproteins impact the innate immune response through Toll-like receptor 2. The acyl chains added to lipoproteins are derived from membrane phospholipids. H. pylori membrane phospholipids have previously been shown to consist primarily of C14:0 and C19:0 cyclopropane-containing acyl chains. However, the acyl composition of H. pylori lipoproteins has not been determined. In this study, we characterized the acyl composition of two representative H. pylori lipoproteins, Lpp20 and CagT. Fatty acid methyl esters were prepared from both purified lipoproteins and analyzed by gas chromatography-mass spectrometry. For comparison, we also analyzed H. pylori phospholipids. Consistent with previous studies, we observed that the H. pylori phospholipids contain primarily C14:0 and C19:0 cyclopropane-containing fatty acids. In contrast, both the ester-linked and amide-linked fatty acids found in H. pylori lipoproteins were observed to be almost exclusively C16:0 and C18:0. A discrepancy between the acyl composition of membrane phospholipids and lipoproteins as reported here for H. pylori has been previously reported in other bacteria including Borrelia and Brucella. We discuss possible mechanisms.IMPORTANCEColonization of the stomach by Helicobacter pylori is an important risk factor in the development of gastric cancer, the third leading cause of cancer-related death worldwide. H. pylori persists in the stomach despite an immune response against the bacteria. Recognition of lipoproteins by TLR2 contributes to the innate immune response to H. pylori. However, the role of H. pylori lipoproteins in bacterial persistence is poorly understood. As the host response to lipoproteins depends on the acyl chain content, defining the acyl composition of H. pylori lipoproteins is an important step in characterizing how lipoproteins contribute to persistence.
Keywords: Helicobacter pylori; Toll-like receptor 2; acyltransferases; lipoproteins; phospholipids; posttranslational protein modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Essential role of Helicobacter pylori apolipoprotein N-acyltransferase (Lnt) in stomach colonization.Infect Immun. 2023 Dec 12;91(12):e0036923. doi: 10.1128/iai.00369-23. Epub 2023 Nov 8. Infect Immun. 2023. PMID: 37937999 Free PMC article.
-
Lipoprotein Processing and Sorting in Helicobacter pylori.mBio. 2020 May 19;11(3):e00911-20. doi: 10.1128/mBio.00911-20. mBio. 2020. PMID: 32430470 Free PMC article.
-
The Cyclopropane Fatty Acid Synthase Mediates Antibiotic Resistance and Gastric Colonization of Helicobacter pylori.J Bacteriol. 2019 Sep 20;201(20):e00374-19. doi: 10.1128/JB.00374-19. Print 2019 Oct 15. J Bacteriol. 2019. PMID: 31358615 Free PMC article.
-
Strategies used by helicobacter pylori to establish persistent infection.World J Gastroenterol. 2017 Apr 28;23(16):2870-2882. doi: 10.3748/wjg.v23.i16.2870. World J Gastroenterol. 2017. PMID: 28522905 Free PMC article. Review.
-
Structural modifications of Helicobacter pylori lipopolysaccharide: an idea for how to live in peace.World J Gastroenterol. 2014 Aug 7;20(29):9882-97. doi: 10.3748/wjg.v20.i29.9882. World J Gastroenterol. 2014. PMID: 25110419 Free PMC article. Review.
Cited by
-
Challenges and Prospects for Eradication of Helicobacter pylori: Targeting Virulence Factors, Metabolism, and Vaccine Innovation.Pathogens. 2025 Jun 21;14(7):619. doi: 10.3390/pathogens14070619. Pathogens. 2025. PMID: 40732667 Free PMC article. Review.
-
Species-specific components of the Helicobacter pylori Cag type IV secretion system.Infect Immun. 2025 May 13;93(5):e0049324. doi: 10.1128/iai.00493-24. Epub 2025 Apr 10. Infect Immun. 2025. PMID: 40208031 Free PMC article.
References
-
- Lai SH, Philbrick WM, Wu HC. 1980. Acyl moieties in phospholipids are the precursors for the fatty acids in murein lipoprotein of Escherichia coli. J Biol Chem 255:5384–5387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

