Irgm proteins attenuate inflammatory disease in mouse models of genital Chlamydia infection
- PMID: 38501887
- PMCID: PMC11005385
- DOI: 10.1128/mbio.00303-24
Irgm proteins attenuate inflammatory disease in mouse models of genital Chlamydia infection
Abstract
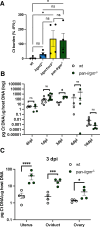
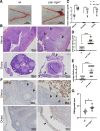
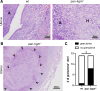
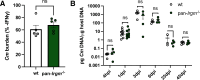
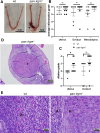
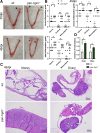
Chlamydiae are obligate intracellular bacterial pathogens that may cause genital pathology via induction of destructive host immune responses. Human-adapted Chlamydia trachomatis causes inflammatory disease in human hosts but is easily cleared in mice, and mouse-adapted Chlamydia muridarum establishes a productive and pathogenic infection in murine hosts. While numerous anti-chlamydial host resistance factors have been discovered in mice and humans alike, little is known about host factors promoting host fitness independent of host resistance. Here, we show that interferon-inducible immunity-related GTPase M (Irgm) proteins function as such host factors ameliorating infection-associated sequalae in the murine female genital tract, thus characterizing Irgm proteins as mediators of disease tolerance. Specifically, we demonstrate that mice deficient for all three murine Irgm paralogs (pan-Irgm-/-) are defective for cell-autonomous immunity to C. trachomatis, which correlates with an early and transient increase in bacterial burden and sustained hyperinflammation in vivo. In contrast, upon infection of pan-Irgm-/- mice with C. muridarum, bacterial burden is unaffected, yet genital inflammation and scarring pathology are nonetheless increased, demonstrating that Irgm proteins can promote host fitness without altering bacterial burden. Additionally, pan-Irgm-/- mice display increased granulomatous inflammation in genital Chlamydia infection, implicating Irgm proteins in the regulation of granuloma formation and maintenance. These findings demonstrate that Irgm proteins regulate pathogenic immune responses to Chlamydia infection in vivo, establishing an effective infection model to examine the immunoregulatory functions and mechanisms of Irgm proteins.
Importance: In response to genital Chlamydia infection, the immune system mounts a proinflammatory response to resist the pathogen, yet inflammation must be tightly controlled to avoid collateral damage and scarring to host genital tissue. Variation in the human IRGM gene is associated with susceptibility to autoinflammatory diseases but its role in ameliorating inflammatory diseases caused by infections is poorly defined. Here, we use mice deficient for all three murine Irgm paralogs to demonstrate that Irgm proteins not only provide host resistance to Chlamydia infections but also limit associated inflammation in the female genital tract. In particular, we find that murine Irgm expression prevents granulomatous inflammation, which parallels inflammatory diseases associated with variants in human IRGM. Our findings therefore establish genital Chlamydia infection as a useful model to study the roles for Irgm proteins in both promoting protective immunity and limiting pathogenic inflammation.
Keywords: Chlamydia; IRGM; disease tolerance; immunity-related GTPases; immunopathology; interferons; sexually transmitted diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Active Hexose-Correlated Compound Restores Gene Expression and Protein Secretion of Protective Cytokines of Immune Cells in a Murine Stress Model during Chlamydia muridarum Genital Infection.Infect Immun. 2021 Apr 16;89(5):e00786-20. doi: 10.1128/IAI.00786-20. Print 2021 Apr 16. Infect Immun. 2021. PMID: 33558321 Free PMC article.
-
Proinflammatory Cytokines in Women with PCOS in Atypical Pathogen Infections.Diagnostics (Basel). 2025 Jun 30;15(13):1669. doi: 10.3390/diagnostics15131669. Diagnostics (Basel). 2025. PMID: 40647668 Free PMC article.
-
Interventions for treating genital Chlamydia trachomatis infection in pregnancy.Cochrane Database Syst Rev. 2017 Sep 22;9(9):CD010485. doi: 10.1002/14651858.CD010485.pub2. Cochrane Database Syst Rev. 2017. PMID: 28937705 Free PMC article.
-
A group B streptococcal type VII-secreted LXG toxin mediates interbacterial competition and colonization of the murine female genital tract.mBio. 2024 Oct 16;15(10):e0208824. doi: 10.1128/mbio.02088-24. Epub 2024 Aug 27. mBio. 2024. PMID: 39189749 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Chlamydia trachomatis Serovars from the C-Complex and the B- and C-Related Complexes Are Significantly More Pathogenic than Those from the B-Complex in C3H/HeN but Not in BALB/c Mice.Pathogens. 2025 Jan 19;14(1):97. doi: 10.3390/pathogens14010097. Pathogens. 2025. PMID: 39861058 Free PMC article.
-
Restriction and evasion: a review of IFNγ-mediated cell-autonomous defense pathways during genital Chlamydia infection.Pathog Dis. 2024 Feb 7;82:ftae019. doi: 10.1093/femspd/ftae019. Pathog Dis. 2024. PMID: 39210512 Free PMC article. Review.
-
Development and Validation of Chlamydia muridarum Mouse Models for Studying Genital Tract Infection Pathogenesis.Bio Protoc. 2025 Feb 5;15(3):e5181. doi: 10.21769/BioProtoc.5181. eCollection 2025 Feb 5. Bio Protoc. 2025. PMID: 39959295 Free PMC article.
References
-
- Braxton JDD, Emerson B. 2018. Sexually transmitted surveillance 2017. Centers for disease control and prevention
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

