Impact of diagnostic investigations in the management of CAR T-cell-associated neurotoxicity
- PMID: 38501964
- PMCID: PMC11131053
- DOI: 10.1182/bloodadvances.2023011669
Impact of diagnostic investigations in the management of CAR T-cell-associated neurotoxicity
Abstract
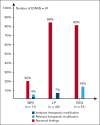
International guidelines regarding the management of immune effector cell-associated neurotoxicity syndrome (ICANS) recommend several diagnostic investigations, including magnetic resonance imaging (MRI), lumbar puncture (LP), and electroencephalogram (EEG) based on ICANS grade. However, the impact of these investigations has not yet been evaluated. Here, we aimed to describe the role of MRI, LP, and EEG in the management of ICANS in a cohort of real-life patients treated with chimeric antigen receptor (CAR) T cells at the University Hospital of Rennes, France. Between August 2018 and January 2023, a total of 190 consecutive patients were treated with CAR T cells. Among those, 91 (48%) developed ICANS. MRI was performed in 71 patients (78%) with ICANS, with a therapeutic impact in 4% of patients, despite frequent abnormal findings. LP was performed in 43 patients (47%), which led to preemptive antimicrobial agents in 7% of patients, although no infection was eventually detected. Systematic EEG was performed in 51 patients (56%), which led to therapeutic modifications in 16% of patients. Our study shows that EEG is the diagnostic investigation with the greatest therapeutic impact, whereas MRI and LP appear to have a limited therapeutic impact. Our results emphasize the role of EEG in the current guidelines but question the need for systematic MRI and LP, which might be left to the discretion of the treating physician.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.H. reports honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, and Roche; and consultancy at Kite/Gilead, Novartis, Bristol Myers Squibb/Celgene, ADC Therapeutics, Incyte, and Miltenyi. G.M. reports honoraria from Bristol Myers Squibb/Celgene, Gilead-Kite, and Takeda. The remaining authors declare no competing financial interests.
Figures
References
-
- Schmidts A, Wehrli M, Maus MV. Toward better understanding and management of CAR-T cell-associated toxicity. Annu Rev Med. 2021;72:365–382. - PubMed
-
- Lowe KL, Mackall CL, Norry E, Amado R, Jakobsen BK, Binder G. Fludarabine and neurotoxicity in engineered T-cell therapy. Gene Ther. 2018;25(3):176–191. - PubMed
-
- Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019;111(7):646–654. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical