ATG-101 Is a Tetravalent PD-L1×4-1BB Bispecific Antibody That Stimulates Antitumor Immunity through PD-L1 Blockade and PD-L1-Directed 4-1BB Activation
- PMID: 38501978
- PMCID: PMC11094422
- DOI: 10.1158/0008-5472.CAN-23-2701
ATG-101 Is a Tetravalent PD-L1×4-1BB Bispecific Antibody That Stimulates Antitumor Immunity through PD-L1 Blockade and PD-L1-Directed 4-1BB Activation
Abstract
Immune checkpoint inhibitors (ICI) have transformed cancer treatment. However, only a minority of patients achieve a profound response. Many patients are innately resistant while others acquire resistance to ICIs. Furthermore, hepatotoxicity and suboptimal efficacy have hampered the clinical development of agonists of 4-1BB, a promising immune-stimulating target. To effectively target 4-1BB and treat diseases resistant to ICIs, we engineered ATG-101, a tetravalent "2+2″ PD-L1×4-1BB bispecific antibody. ATG-101 bound PD-L1 and 4-1BB concurrently, with a greater affinity for PD-L1, and potently activated 4-1BB+ T cells when cross-linked with PD-L1-positive cells. ATG-101 activated exhausted T cells upon PD-L1 binding, indicating a possible role in reversing T-cell dysfunction. ATG-101 displayed potent antitumor activity in numerous in vivo tumor models, including those resistant or refractory to ICIs. ATG-101 greatly increased the proliferation of CD8+ T cells, the infiltration of effector memory T cells, and the ratio of CD8+ T/regulatory T cells in the tumor microenvironment (TME), rendering an immunologically "cold" tumor "hot." Comprehensive characterization of the TME after ATG-101 treatment using single-cell RNA sequencing further revealed an altered immune landscape that reflected increased antitumor immunity. ATG-101 was well tolerated and did not induce hepatotoxicity in non-human primates. According to computational semimechanistic pharmacology modeling, 4-1BB/ATG-101/PD-L1 trimer formation and PD-L1 receptor occupancy were both maximized at around 2 mg/kg of ATG-101, providing guidance regarding the optimal biological dose for clinical trials. In summary, by localizing to PD-L1-rich microenvironments and activating 4-1BB+ immune cells in a PD-L1 cross-linking-dependent manner, ATG-101 safely inhibits growth of ICI resistant and refractory tumors.
Significance: The tetravalent PD-L1×4-1BB bispecific antibody ATG-101 activates 4-1BB+ T cells in a PD-L1 cross-linking-dependent manner, minimizing the hepatotoxicity of existing 4-1BB agonists and suppressing growth of ICI-resistant tumors. See related commentary by Ha et al., p. 1546.
©2024 The Authors; Published by the American Association for Cancer Research.
Figures
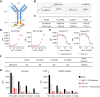
![Figure 2. ATG-101 activates 4-1BB signaling upon PD-L1 cross-linking. A and B, The 4-1BB agonistic activity of ATG-101 in NFκB luciferase assay. A, 293T-h41BB-NFκB-Luc cells were incubated with ATG-101 and CHO-hPDL1 (left) or CHO-mPDL1 (right) for 24 hours. Wild-type CHO cells as negative control. B, 4-1BB activation by ATG-101, bivalent PD-L1×4-1BB bsAb [ATG-101(1+1)], or isotype control antibodies in 293T-h41BB-NFκB-Luc cells with the presence of 293T-hPDL1. Tetravalent and bivalent ATG-101 activated 4-1BB signal pathway with EC50 of 5.25 nmol/L and 15.44 nmol/L, respectively. C, Effect of ATG-101 on IL2 release by CD8+ T cells with the presence of PD-L1+ cell lines. PD-L1 densities (total antibody binding sites) on MCF7, RKO, NCI-H358, and 293T-hPDL1 cell surfaces were determined using QIFIKIT (left). All the indicated cells cocultured with CD8+ T cells and ATG-101 for 3 days, and supernatants were harvested for IL2 ELISA to assess T-cell activation (right). D, ATG-101–induced IFNγ release by human primary CD8+ T cells coculturing with varying proportions of PD-L1+ cells. HEK293-PDL1 cells were mixed with parental HEK293 at different proportions. The IFNγ release was detected by ELISA. E, The individual and mean expression pattern of surface markers and cytokine release of CD3+ T cells from three healthy donors through six rounds of CD3/CD28 activation. F, Individual and mean IL2, IFNγ, and TNFα production by T cells after six rounds of activation (from E), in response to ATG-101, parental PD-L1 antibody, parental 4-1BB antibody, or isotype control. In A, C, and D, data representative of three independent experiments. In A, B, C, and F, data are presented as means ± SEM. Statistical analysis used two-way ANOVA in C and one-way ANOVA in F. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/453f/11094422/23d03aafca93/1680fig2.gif)




References
-
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–30. - PubMed
-
- Sarmento-Ribeiro AB, Scorilas A, Goncalves AC, Efferth T, Trougakos IP. The emergence of drug resistance to targeted cancer therapies: clinical evidence. Drug Resist Updat 2019;47:100646. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

