Linkage of individual-patient data confirm protection of prophylactic human papillomavirus vaccination against invasive cervical cancer
- PMID: 38501990
- PMCID: PMC11160490
- DOI: 10.1093/jnci/djae042
Linkage of individual-patient data confirm protection of prophylactic human papillomavirus vaccination against invasive cervical cancer
Figures
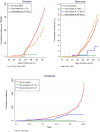
Comment on
-
Invasive cervical cancer incidence following bivalent human papillomavirus vaccination: a population-based observational study of age at immunization, dose, and deprivation.J Natl Cancer Inst. 2024 Jun 7;116(6):857-865. doi: 10.1093/jnci/djad263. J Natl Cancer Inst. 2024. PMID: 38247547
References
-
- Pagliusi SR, Teresa AM.. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine. 2004;23(5):569-578. - PubMed
-
- IARC, NCI. Primary End-points for Prophylactic HPV Vaccine Trials IARC Working Group Report. Vol. 7, 2014. https://www.iarc.who.int/wp-content/uploads/2018/07/Prophylactic_HPV_Vac... - PubMed

