Synthesis and Photophysical Properties of β-Alkenyl-Substituted BODIPY Dyes by Indium(III)-Catalyzed Intermolecular Alkyne Hydroarylation
- PMID: 38502009
- PMCID: PMC11002825
- DOI: 10.1021/acs.joc.3c02951
Synthesis and Photophysical Properties of β-Alkenyl-Substituted BODIPY Dyes by Indium(III)-Catalyzed Intermolecular Alkyne Hydroarylation
Abstract
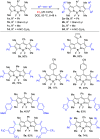
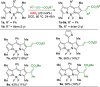
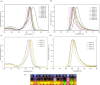
A new atom-economical synthesis of β-alkenyl-substituted BODIPYs via indium(III)-catalyzed intermolecular alkyne hydroarylation with meso-substituted BODIPYs is described. While catalysis with InI3 allows the double β-functionalization of BODIPY, resulting in regioselectively branched β,β'-disubstituted alkenyl BODIPYs, catalytic InCl3 enables the formation of linear β-substituted alkenyl BODIPYs. Subsequent In(III)-catalyzed intermolecular alkyne hydroarylation allows the synthesis of unsymmetrical push-pull BODIPY derivatives. Therefore, indium catalysis offers complementary regioselectivity in good chemical yields and functional group tolerance. The resulting BODIPY dyes displayed bathochromically shifted absorption and emission according to the electron-nature of the substituents in the alkenyl moiety with high molar extinction coefficients (ε up to 88,200 M-1 cm-1) and quantum yields (0.14-0.96).
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Ni Y.; Wu J. Far-Red and near Infrared BODIPY Dyes: Synthesis and Applications for Fluorescent pH Probes and Bio-Imaging. Org. Biomol. Chem. 2014, 12, 3774–3791. 10.1039/c3ob42554a. - DOI - PubMed
- Samanta S.; Lai K.; Wu F.; Liu Y.; Cai S.; Yang X.; Qu J.; Yang Z. Xanthene, Cyanine, Oxazine and BODIPY: The Four Pillars of the Fluorophore Empire for Super-Resolution Bioimaging. Chem. Soc. Rev. 2023, 52, 7197–7261. 10.1039/D2CS00905F. - DOI - PubMed
-
- Awuah S. G.; You Y. Boron Dipyrromethene (BODIPY)-Based Photosensitizers for Photodynamic Therapy. RSC Adv. 2012, 2, 11169–11183. 10.1039/c2ra21404k. - DOI
- Kamkaew A.; Lim S. H.; Lee H. B.; Kiew L. V.; Chung L. Y.; Burgess K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. 10.1039/C2CS35216H. - DOI - PMC - PubMed
- Bassan E.; Gualandi A.; Cozzi P. G.; Ceroni P. Design of BODIPY Dyes as Triplet Photosensitizers: Electronic Properties Tailored for Solar Energy Conversion, Photoredox Catalysis and Photodynamic Therapy. Chem. Sci. 2021, 12, 6607–6628. 10.1039/D1SC00732G. - DOI - PMC - PubMed
-
- Rana P.; Singh N.; Majumdar P.; Singh S. P. Evolution of BODIPY/Aza-BODIPY Dyes for Organic Photoredox/Energy Transfer Catalysis. Coord. Chem. Rev. 2022, 470, 214698.10.1016/j.ccr.2022.214698. - DOI
-
- Singh S. P.; Gayathri T. Evolution of BODIPY Dyes as Potential Sensitizers for Dye-Sensitized Solar Cells. Eur. J. Org Chem. 2014, 2014, 4689–4707. 10.1002/ejoc.201400093. - DOI
LinkOut - more resources
Full Text Sources

