Giants among Cnidaria: Large Nuclear Genomes and Rearranged Mitochondrial Genomes in Siphonophores
- PMID: 38502059
- PMCID: PMC10980510
- DOI: 10.1093/gbe/evae048
Giants among Cnidaria: Large Nuclear Genomes and Rearranged Mitochondrial Genomes in Siphonophores
Abstract
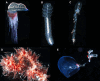
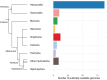
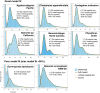
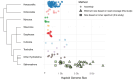
Siphonophores (Cnidaria: Hydrozoa) are abundant predators found throughout the ocean and are important constituents of the global zooplankton community. They range in length from a few centimeters to tens of meters. They are gelatinous, fragile, and difficult to collect, so many aspects of the biology of these roughly 200 species remain poorly understood. To survey siphonophore genome diversity, we performed Illumina sequencing of 32 species sampled broadly across the phylogeny. Sequencing depth was sufficient to estimate nuclear genome size from k-mer spectra in six specimens, ranging from 0.7 to 2.3 Gb, with heterozygosity estimates between 0.69% and 2.32%. Incremental k-mer counting indicates k-mer peaks can be absent with nearly 20× read coverage, suggesting minimum genome sizes range from 1.4 to 5.6 Gb in the 25 samples without peaks in the k-mer spectra. This work confirms most siphonophore nuclear genomes are large relative to the genomes of other cnidarians, but also identifies several with reduced size that are tractable targets for future siphonophore nuclear genome assembly projects. We also assembled complete mitochondrial genomes for 33 specimens from these new data, indicating a conserved gene order shared among nonsiphonophore hydrozoans, Cystonectae, and some Physonectae, revealing the ancestral mitochondrial gene order of siphonophores. Our results also suggest extensive rearrangement of mitochondrial genomes within other Physonectae and in Calycophorae. Though siphonophores comprise a small fraction of cnidarian species, this survey greatly expands our understanding of cnidarian genome diversity. This study further illustrates both the importance of deep phylogenetic sampling and the utility of k-mer-based genome skimming in understanding the genomic diversity of a clade.
Keywords: k-mer spectra; genome size; genome skimming; mitochondrial genomes.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures


References
-
- Adachi K, Miyake H, Kuramochi T, Mizusawa K, Okumura S-I. Genome size distribution in phylum Cnidaria. Fish Sci. 2017:83(1):107–112. 10.1007/s12562-016-1050-4. - DOI
-
- Bargmann HE, Totton AK. A synopsis of the Siphonophora. By A.K. Totton assisted by H.E. Bargmann, etc. London: Trustees of the British Museum (Natural History); 1965.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

