Stroke Risk After COVID-19 Bivalent Vaccination Among US Older Adults
- PMID: 38502075
- PMCID: PMC10951737
- DOI: 10.1001/jama.2024.1059
Stroke Risk After COVID-19 Bivalent Vaccination Among US Older Adults
Abstract
Importance: In January 2023, the US Centers for Disease Control and Prevention and the US Food and Drug Administration noted a safety concern for ischemic stroke among adults aged 65 years or older who received the Pfizer-BioNTech BNT162b2; WT/OMI BA.4/BA.5 COVID-19 bivalent vaccine.
Objective: To evaluate stroke risk after administration of (1) either brand of the COVID-19 bivalent vaccine, (2) either brand of the COVID-19 bivalent plus a high-dose or adjuvanted influenza vaccine on the same day (concomitant administration), and (3) a high-dose or adjuvanted influenza vaccine.
Design, setting, and participants: Self-controlled case series including 11 001 Medicare beneficiaries aged 65 years or older who experienced stroke after receiving either brand of the COVID-19 bivalent vaccine (among 5 397 278 vaccinated individuals). The study period was August 31, 2022, through February 4, 2023.
Exposures: Receipt of (1) either brand of the COVID-19 bivalent vaccine (primary) or (2) a high-dose or adjuvanted influenza vaccine (secondary).
Main outcomes and measures: Stroke risk (nonhemorrhagic stroke, transient ischemic attack, combined outcome of nonhemorrhagic stroke or transient ischemic attack, or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day risk window after vaccination vs the 43- to 90-day control window.
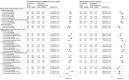
Results: There were 5 397 278 Medicare beneficiaries who received either brand of the COVID-19 bivalent vaccine (median age, 74 years [IQR, 70-80 years]; 56% were women). Among the 11 001 beneficiaries who experienced stroke after receiving either brand of the COVID-19 bivalent vaccine, there were no statistically significant associations between either brand of the COVID-19 bivalent vaccine and the outcomes of nonhemorrhagic stroke, transient ischemic attack, nonhemorrhagic stroke or transient ischemic attack, or hemorrhagic stroke during the 1- to 21-day or 22- to 42-day risk window vs the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12). Among the 4596 beneficiaries who experienced stroke after concomitant administration of either brand of the COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine, there was a statistically significant association between vaccination and nonhemorrhagic stroke during the 22- to 42-day risk window for the Pfizer-BioNTech BNT162b2; WT/OMI BA.4/BA.5 COVID-19 bivalent vaccine (IRR, 1.20 [95% CI, 1.01-1.42]; risk difference/100 000 doses, 3.13 [95% CI, 0.05-6.22]) and a statistically significant association between vaccination and transient ischemic attack during the 1- to 21-day risk window for the Moderna mRNA-1273.222 COVID-19 bivalent vaccine (IRR, 1.35 [95% CI, 1.06-1.74]; risk difference/100 000 doses, 3.33 [95% CI, 0.46-6.20]). Among the 21 345 beneficiaries who experienced stroke after administration of a high-dose or adjuvanted influenza vaccine, there was a statistically significant association between vaccination and nonhemorrhagic stroke during the 22- to 42-day risk window (IRR, 1.09 [95% CI, 1.02-1.17]; risk difference/100 000 doses, 1.65 [95% CI, 0.43-2.87]).
Conclusions and relevance: Among Medicare beneficiaries aged 65 years or older who experienced stroke after receiving either brand of the COVID-19 bivalent vaccine, there was no evidence of a significantly elevated risk for stroke during the days immediately after vaccination.
Conflict of interest statement
Figures



Comment in
-
Postmarketing Vaccine Safety Assessments: Important Work in Progress.JAMA. 2024 Mar 19;331(11):915-917. doi: 10.1001/jama.2023.26630. JAMA. 2024. PMID: 38502085 No abstract available.
References
-
- US Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. Published August 31, 2022. Accessed February 21, 2024. https://www.fda.gov/emergency-preparedness-and-response/counterterrorism...
-
- US Food and Drug Administration . FDA takes action on updated mRNA COVID-19 vaccines to better protect against currently circulating variants. Published September 11, 2023. Accessed February 21, 2024. https://www.fda.gov/news-events/press-announcements/fda-takes-action-upd...
-
- US Food and Drug Administration . CDC and FDA identify preliminary COVID-19 vaccine safety signal for persons aged 65 years and older. Updated May 31, 2023. Accessed February 21, 2024. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologi...
-
- Protection of Human Subjects, 45 CFR Part 46 and §46.104(d)(2).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

