WWP2 Regulates Renal Fibrosis and the Metabolic Reprogramming of Profibrotic Myofibroblasts
- PMID: 38502123
- PMCID: PMC11164121
- DOI: 10.1681/ASN.0000000000000328
WWP2 Regulates Renal Fibrosis and the Metabolic Reprogramming of Profibrotic Myofibroblasts
Abstract
Key Points:
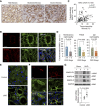
WWP2 expression is elevated in the tubulointerstitium of fibrotic kidneys and contributes to CKD pathogenesis and progression.
WWP2 uncouples the profibrotic activation and cell proliferation in renal myofibroblasts.
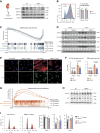
WWP2 controls mitochondrial respiration in renal myofibroblasts through the metabolic regulator peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Background: Renal fibrosis is a common pathologic end point in CKD that is challenging to reverse, and myofibroblasts are responsible for the accumulation of a fibrillar collagen–rich extracellular matrix. Recent studies have unveiled myofibroblasts' diversity in proliferative and fibrotic characteristics, which are linked to different metabolic states. We previously demonstrated the regulation of extracellular matrix genes and tissue fibrosis by WWP2, a multifunctional E3 ubiquitin–protein ligase. Here, we investigate WWP2 in renal fibrosis and in the metabolic reprograming of myofibroblasts in CKD.
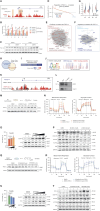
Methods: We used kidney samples from patients with CKD and WWP2-null kidney disease mice models and leveraged single-cell RNA sequencing analysis to detail the cell-specific regulation of WWP2 in fibrotic kidneys. Experiments in primary cultured myofibroblasts by bulk-RNA sequencing, chromatin immunoprecipitation sequencing, metabolomics, and cellular metabolism assays were used to study the metabolic regulation of WWP2 and its downstream signaling.
Results: The tubulointerstitial expression of WWP2 was associated with fibrotic progression in patients with CKD and in murine kidney disease models. WWP2 deficiency promoted myofibroblast proliferation and halted profibrotic activation, reducing the severity of renal fibrosis in vivo. In renal myofibroblasts, WWP2 deficiency increased fatty acid oxidation and activated the pentose phosphate pathway, boosting mitochondrial respiration at the expense of glycolysis. WWP2 suppressed the transcription of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a metabolic mediator of fibrotic response, and pharmacologic inhibition of PGC-1α partially abrogated the protective effects of WWP2 deficiency on myofibroblasts.
Conclusions: WWP2 regulates the metabolic reprogramming of profibrotic myofibroblasts by a WWP2-PGC-1α axis, and WWP2 deficiency protects against renal fibrosis in CKD.
Conflict of interest statement
J. Behmoaras and E. Petretto report employment with Duke-NUS Medical School. G. Chew reports employment with Ministry of Health Holdings, Singapore. L. Gesualdo reports consultancy for AstraZeneca, Chinook, GSK, Novartis, Roche, and Travere and research funding from Abionyx, AstraZeneca, and Sanofi. F. Pesce reports honoraria from AstraZeneca and GSK. P. Pontrelli reports employment with University of Bari Aldo Moro, patents or royalties from University of Bari Aldo Moro, and other interests or relationships with ERA and SIN. All remaining authors have nothing to disclose.
Figures




References
-
- Zeisberg M, Strutz F, Muller GA. Role of fibroblast activation in inducing interstitial fibrosis. J Nephrol. 2000;13(suppl 3):S111–S120. PMID: 11132027 - PubMed
MeSH terms
Grants and funding
- T2EP30221-0013/Ministry of Education - Singapore
- 81830020/National Natural Science Foundation of China
- OFLCG22may-0011/National Research Foundation Singapore
- Mission 4, Component 2, Investment 1.4 (CN00000041)/the European Union â€" NextGenerationEU (PNRR)
- Mission 4, Component 2, Investment 1.4 (CN00000041)/the European Union - NextGenerationEU (PNRR)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

